Acetylcholine esterase mutant and application thereof
A technology of acetylcholinesterase and mutants, which is applied in the field of food testing and public health, and can solve the problems of complicated sample processing, inapplicability of rapid detection, and dependence on large-scale detection instruments.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
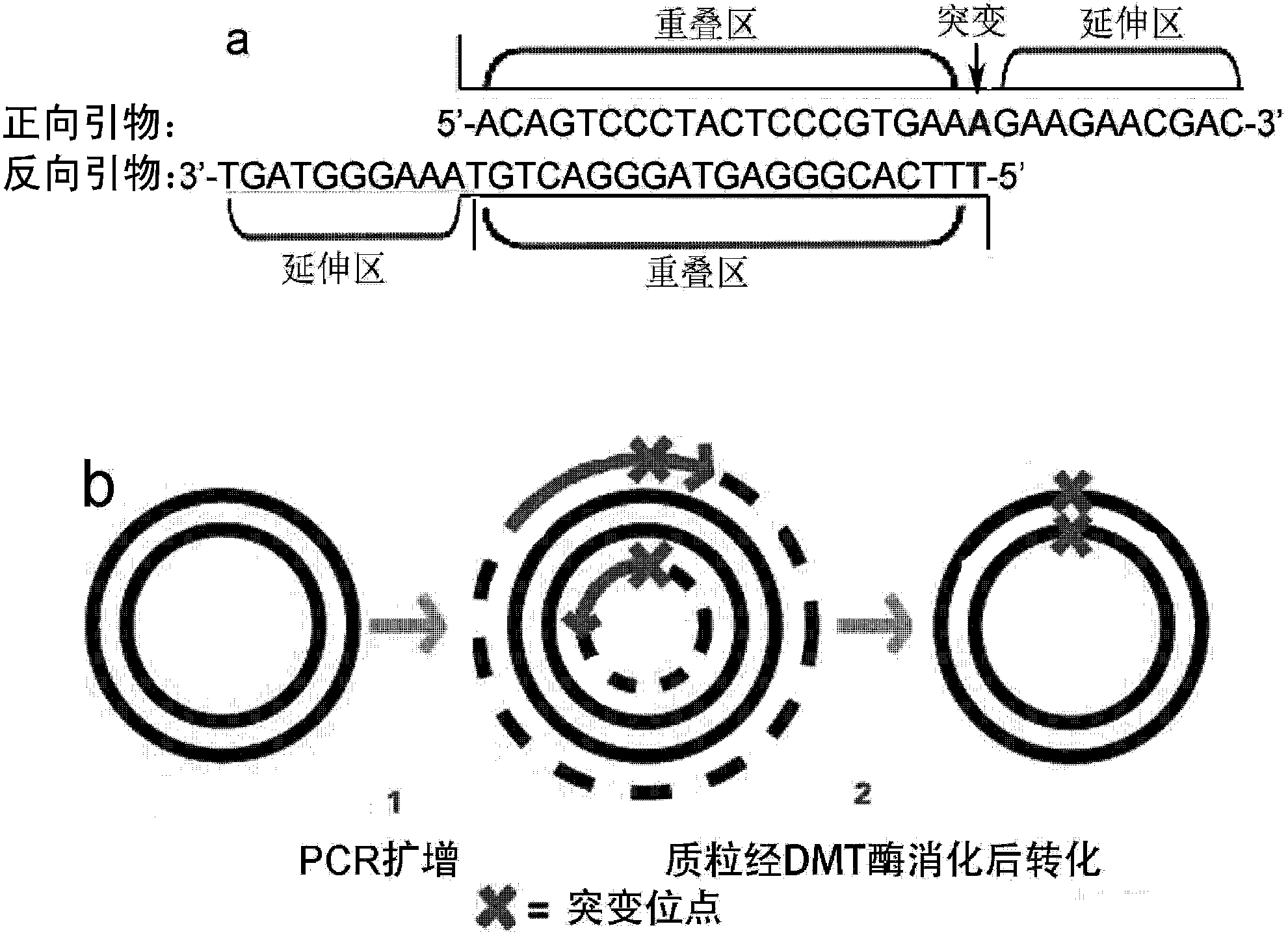
[0111] Embodiment 1, the construction of acetylcholinesterase mutant
[0112] The full length of the acetylcholinesterase (AChE) gene is 1950bp (SEQ ID NO: 1; GenBank accession number: NM_057605), which can encode 649 amino acids (SEQ ID NO: 2). The N-terminal of the protein sequence of the enzyme contains a signal peptide sequence, and its C The end contains its own GPI anchor sequence. The 408th Tyr of the wild-type protein shown in SEQ ID NO: 2 is the mutation site of the present invention.
[0113] In order to display and express on the surface of yeast cells, the inventors carried out a transformation of genetic engineering technology (see patent No. ZL 200810201894.5), and the signal peptide (1-38aa) at the N-terminus was replaced by the glucoamylase secretion signal peptide sequence , the C-terminal anchoring sequence (620-649aa) was replaced by the yeast's own α-lectin gene anchoring sequence, and a Flag epitope was added between the α-lectin gene anchoring sequence a...
Embodiment 2
[0122] Example 2, Induced expression of acetylcholinesterase and determination of enzyme activity
[0123] Transformed yeast cells (including yeast cells expressing mutant acetylcholinesterase and yeast cells expressing wild-type acetylcholinesterase) were screened for transformants on SC selection plates, and single clones were picked and cultured in 5ml SC medium at 30°C Overnight, the yeast cells were harvested by centrifugation, and the resulting yeast cells were induced to express protein, that is, the yeast cells cultured overnight were diluted to OD with SC medium with galactose as the carbon source 600 0.4 cell suspension, induced at 30°C for 4 hours, centrifuged at 10,000rpm at 4°C for 5min to harvest the cells.
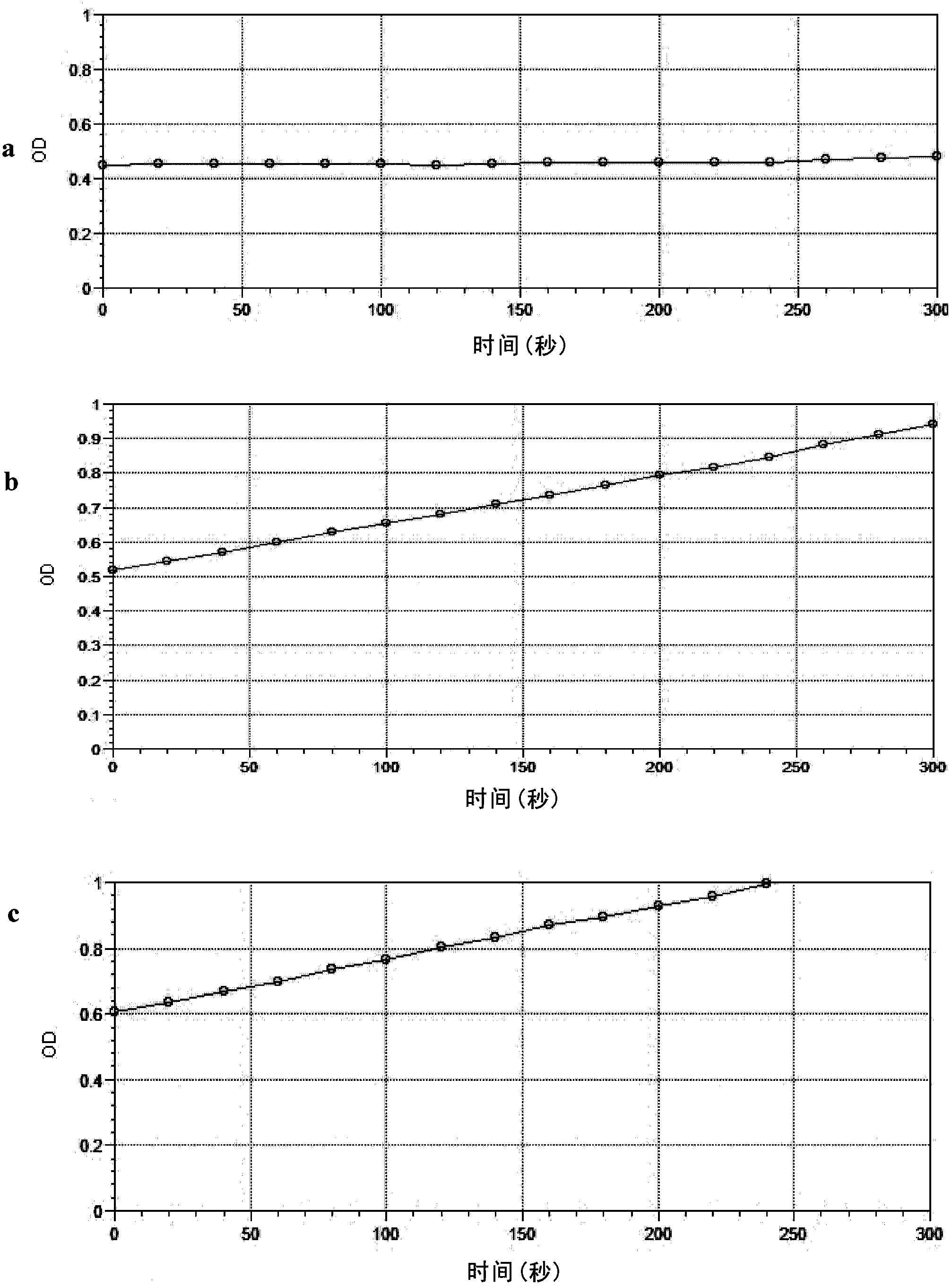
[0124] Cells were resuspended in sodium phosphate buffer (pH 7.6) to make OD 600 After that, the substrate acetylcholine iodide (ATChI) and the chromogenic agent dithiodinitrobenzoic acid (DTNB) were sequentially added at a final concentration of 1 mM, and ...
Embodiment 3
[0128] Embodiment 3, the inhibition of organophosphorus and carbamate pesticides to acetylcholinesterase mutant
[0129] Yeast cells were induced to express mutant acetylcholinesterase or wild-type (non-mutated) acetylcholinesterase, collected by centrifugation, resuspended in sodium phosphate buffer (pH 7.6), added to a 96-well plate, and made OD 600 The value is 0.5, adding different final concentrations of organophosphorus and carbamate pesticides, and incubating at 37°C for 30 minutes to make the pesticides fully inhibit acetylcholine ester, and then adding the substrate ATChI and the display agent DTNB with a final concentration of 1mM, the total reaction system 200μL, react at 37°C for 15min, detect OD every 0.5min during the reaction 412 .
[0130] Taking the data without pesticide inhibition as a control, that is, the retained enzyme activity is 100%, the inhibition degree of 9 kinds of organophosphorus and carbamate pesticides to acetylcholinesterase can be from Fi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com