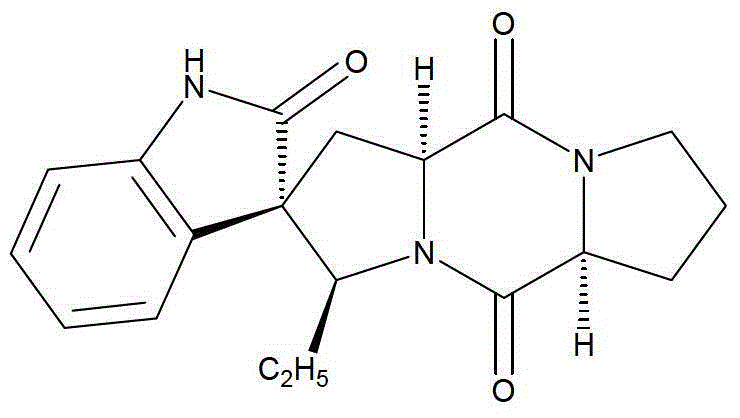
A kind of spirocyclic indole diketopiperazine alkaloid and its synthesis method and application
A technology of indole diketopiperazine and synthesis method, which is applied in the direction of bulk chemical production, organic chemistry, antibacterial drugs, etc., can solve the problems of complex reaction route, cumbersome post-processing, high cost, etc., and achieve the purpose of improving reaction yield, The effect of convenient processing and reduced dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
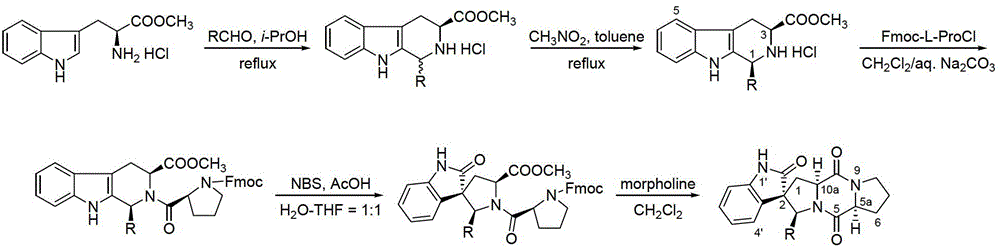
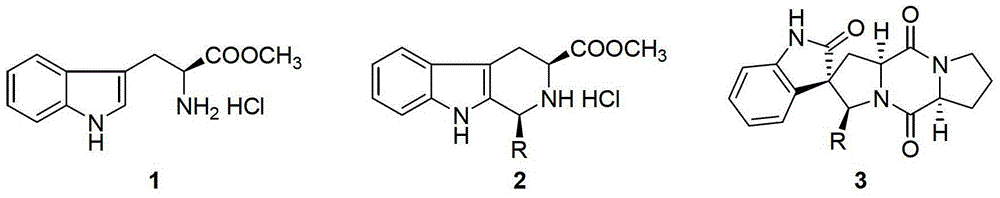
[0033] 1) Add 50mL of isopropanol to a 100mL three-necked flask equipped with a stirrer, a constant pressure dropping funnel, and a condensing reflux device, and then add 12mmol of propionaldehyde into the three-necked flask under stirring, and after mixing evenly, add 10mmol of L-tryptophan methyl ester hydrochloride, then heated to reflux state reaction, carried out with TLC monitoring reaction in the reflux reaction process, reacted 4h, now TLC detects that L-tryptophan methyl ester hydrochloride raw material point disappears and Generate two new product points, end the reaction, evaporate isopropanol and excess propionaldehyde under reduced pressure to obtain solid A, solid A is rinsed with toluene to remove soluble impurities, and then dried to obtain mixed enantiomeric hydrochloride (a cis-trans mixture of mix-2a-HCl); wherein, the TLC developer is a mixture of ethyl acetate and methanol at a volume ratio of 10:1;
[0034] 2) Mix nitromethane and toluene uniformly at a v...
Embodiment 2
[0049] 1) Add about 50mL of isopropanol into a 100mL three-necked flask equipped with a stirrer, a constant pressure dropping funnel, and a condensing reflux device, then add 12mmol of n-butyraldehyde into the three-necked flask under stirring, stir and mix evenly, and then add 10mmol of L-tryptophan methyl ester hydrochloride, then heated to the reflux state to react, during the reflux reaction, use TLC to monitor the reaction, react for 4h, at this time TLC detects the raw material point of L-tryptophan methyl ester hydrochloride Disappear and generate two new product points, end the reaction, evaporate isopropanol and excess n-butyraldehyde under reduced pressure to obtain solid A, solid A is rinsed with toluene to remove soluble impurities, then dried to obtain mixed enantiomers Hydrochloride (mix-2b-HCl cis-trans mixture).
[0050] 2) Mix nitromethane and toluene uniformly at a volume ratio of 1:1 to obtain an inducer for crystallization-induced asymmetric transformation;...
Embodiment 3
[0065] 1) Add 50mL of isopropanol to a 100mL three-necked flask equipped with a stirrer, a constant pressure dropping funnel, and a condensing reflux device, and then add 12mmol of n-valeraldehyde to the three-necked flask while stirring, and after mixing evenly, add 10mmol of L-tryptophan methyl ester hydrochloride, then heated to reflux state reaction, carried out with TLC monitoring reaction in the reflux reaction process, reacted 4h, now TLC detects that L-tryptophan methyl ester hydrochloride raw material point disappears and Generate two new product points, end the reaction, evaporate isopropanol and excess n-valeraldehyde under reduced pressure to obtain solid A, solid A is rinsed with toluene to remove soluble impurities, and then dried to obtain mixed enantiomeric hydrochloric acid Salt (a cis-trans mixture of mix-2c-HCl); wherein, the TLC developer is a mixture of ethyl acetate and methanol at a volume ratio of 10:1;
[0066]2) Mix nitromethane and toluene uniformly ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com