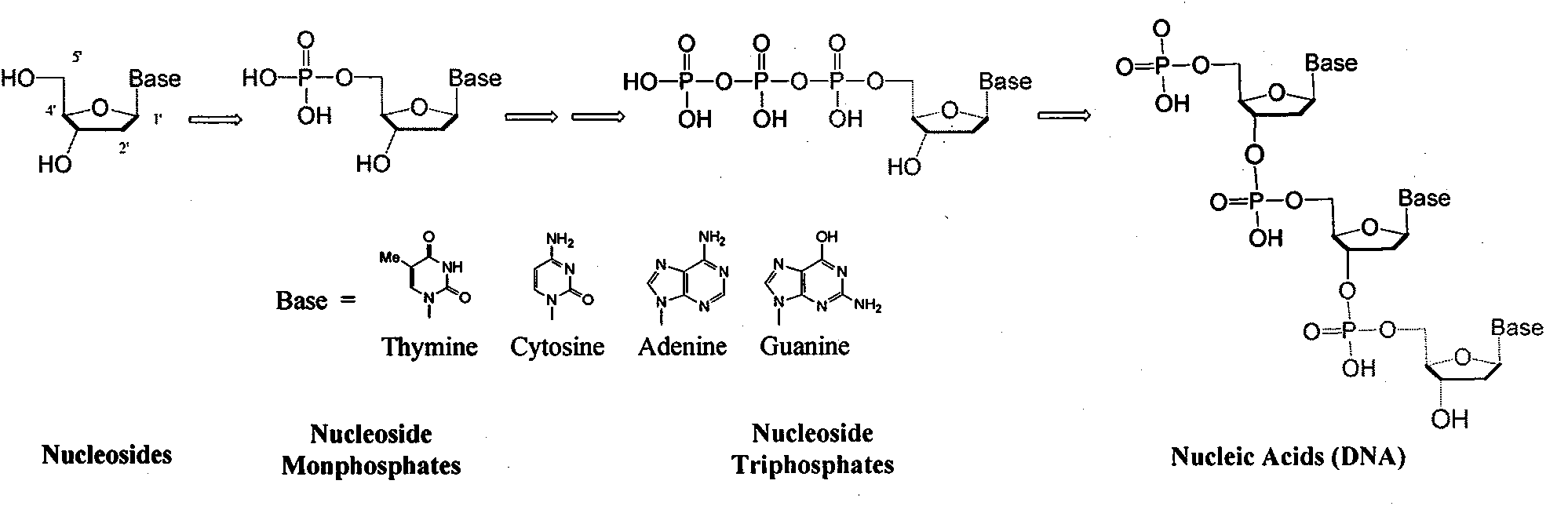
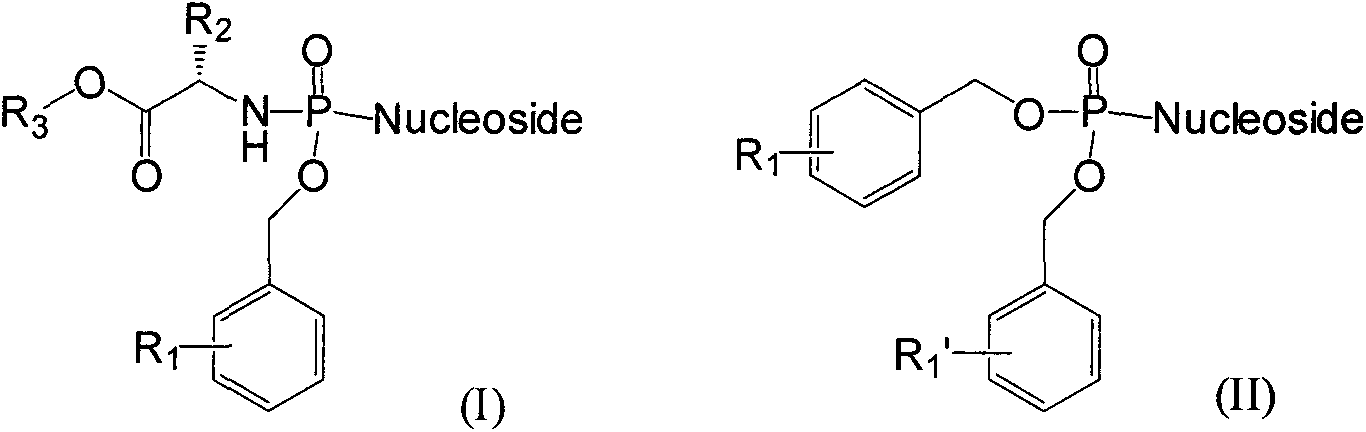
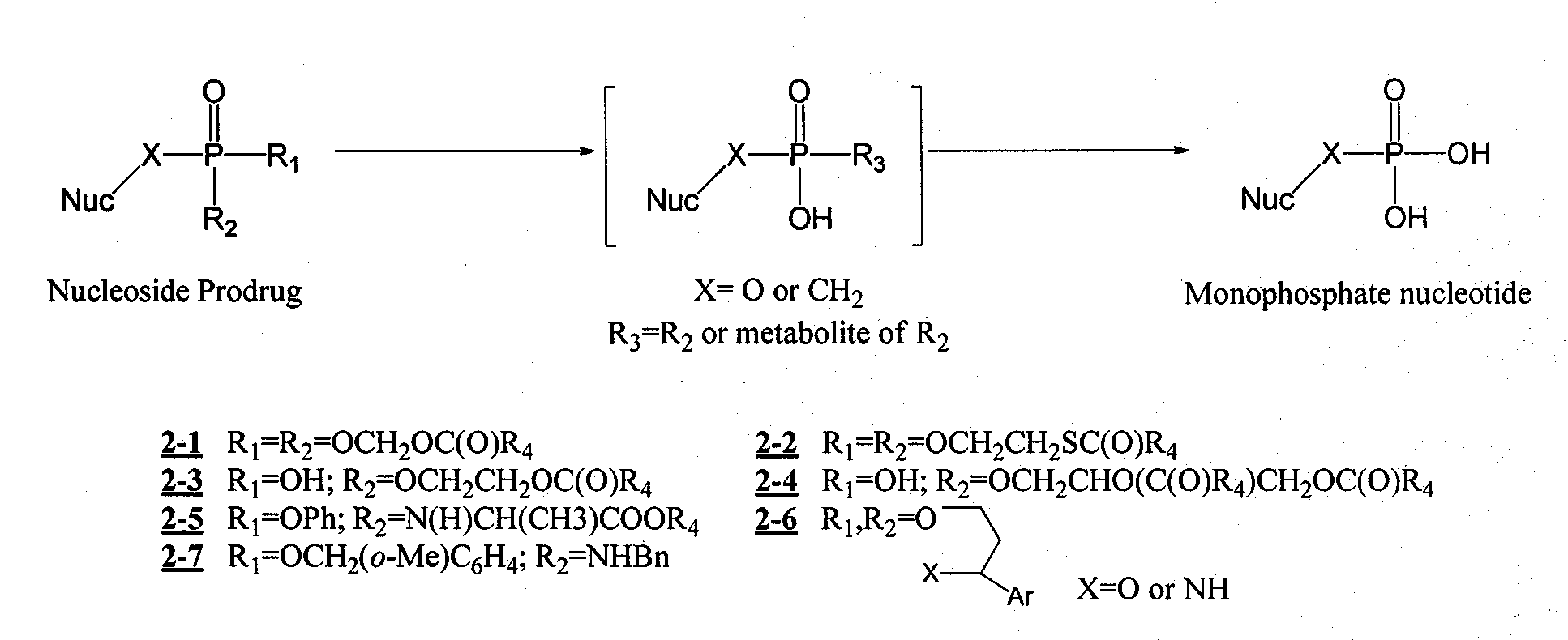Structure and synthesis of novel nucleoside phosphate prodrug containing substituted benzyl
A technology of nucleoside phosphate and phosphate, which is applied in the field of valence and can solve problems such as the inability to use antiviral drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069]
[0070] Compound ( 9 , 12.8g, 50mmol) was dissolved in dichloromethane (100mL) and cooled to -78°C, o-methylbenzyl alcohol (6.1g, 50mmol) and triethylamine (7.7mL, 55mmol) were slowly added dropwise within 20 minutes dichloromethane (100mL) solution. The reaction was stirred at -78 °C for 30 min, then transferred to another reaction containing dry L-alanine hydrochloride (7.68 g, 50 mmol) in dichloromethane (100 mL) at 0 °C in the container. Triethylamine (14.7 mL, 105 mmol) was slowly added dropwise to the above reaction solution, and the dropwise addition was completed in 20 minutes, and the reaction solution was continuously stirred at zero temperature for one hour. The solvent was removed by rotary evaporation, ethyl acetate was added to grind powder, filtered, the filtrate was concentrated, and the residue was separated and purified by silica gel column chromatography (petroleum ether:ethyl acetate=7:3) to obtain a colorless oily product ( 11 ) (17.7g, 84%),...
Embodiment 2
[0073]
[0074] compound ( 12 , 296 mg, 1 mmol) was dissolved in 20 mL of anhydrous tetrahydrofuran, and tert-butylmagnesium chloride Grignard reagent (1.0 M, 4 mL, 4 mmol) was added at room temperature. After stirring and reacting for 30 minutes, slowly add compound ( 11 , 844mg, 2mmol) in tetrahydrofuran (4mL), the reaction mixture was stirred at room temperature for 24 hours, TLC monitored that the reaction was complete, then added saturated ammonium chloride solution (20mL) to quench, extracted with ethyl acetate (20mLx3), the organic phases were combined and dried , concentrated, and the residue was purified by silica gel column chromatography (dichloromethane:methanol=20:1) to obtain a white powder product ( 13 ) (370 mg, 64%). 1 H NMR (CD 3OD, 400MHz) δ8.50(s, 0.5H), 8.47(s, 0.5H), 8.39(s, 0.5H), 8.35(s, 0.5H), 7.28-7.30(m, 1H), 7.09-7.17 (m, 3H), 6.15(s, 0.5H), 6.14(s, 0.5H), 5.04-5.09(m, 2H), 4.22-4.60(m, 4H), 4.16(s, 3H), 4.02-4.07 (m, 2H), 3.78-3.81(m, 1H), ...
Embodiment 3
[0076]
[0077] Using the same synthetic method as in Example 15, the compound ( 14 )and( 11 ) is coupled under the effect of tert-butylmagnesium chloride Grignard reagent to obtain a white powdery product ( 15 ). 1 H NMR (CD 3 OD, 400MHz) δ7.93(s, 0.5H), 7.90(s, 0.5H), 7.28-7.31(m, 1H), 7.10-7.17(m, 3H), 5.95(s, 0.5H), 5.94( s, 0.5H), 5.04-5.09(m, 2H), 4.00-4.43(m, 6H), 4.02(s, 3H), 3.78-3.82(m, 1H), 2.27-2.31(m, 3H), 1.27 -1.31(m, 3H), 1.13-1.16(m, 3H), 0.90-0.95(m, 3H); 31 P NMR (CD 3 OD) δ 9.76, 9.67; MS (m / z) 595 (M+H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com