Novel polymer chiral catalyst, preparation method, and applications thereof
A chiral catalyst and polymer technology, applied in chemical instruments and methods, physical/chemical process catalysts, organic compound/hydride/coordination complex catalysts, etc. Churn and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] A kind of preparation method of polymer chiral catalyst
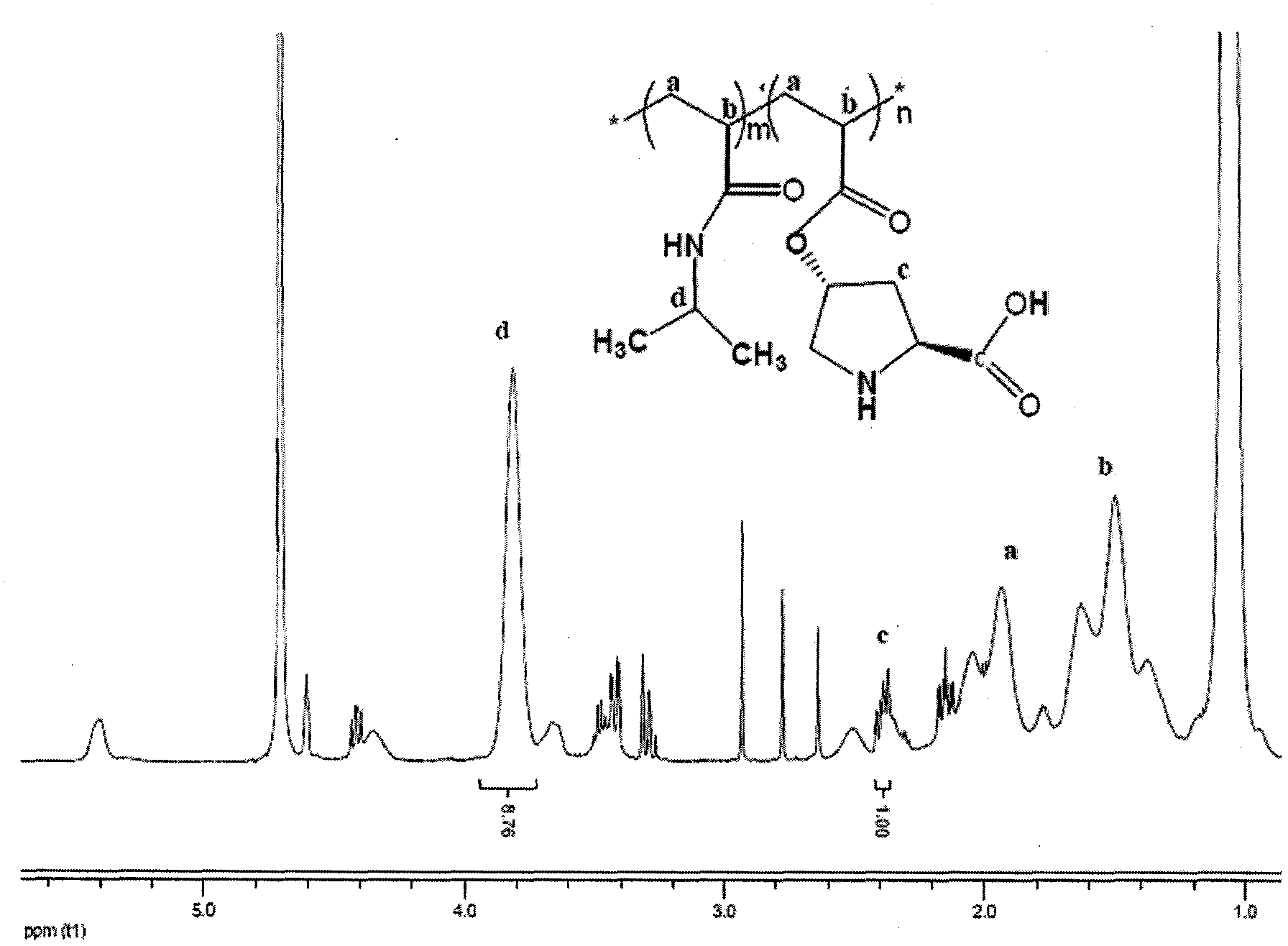
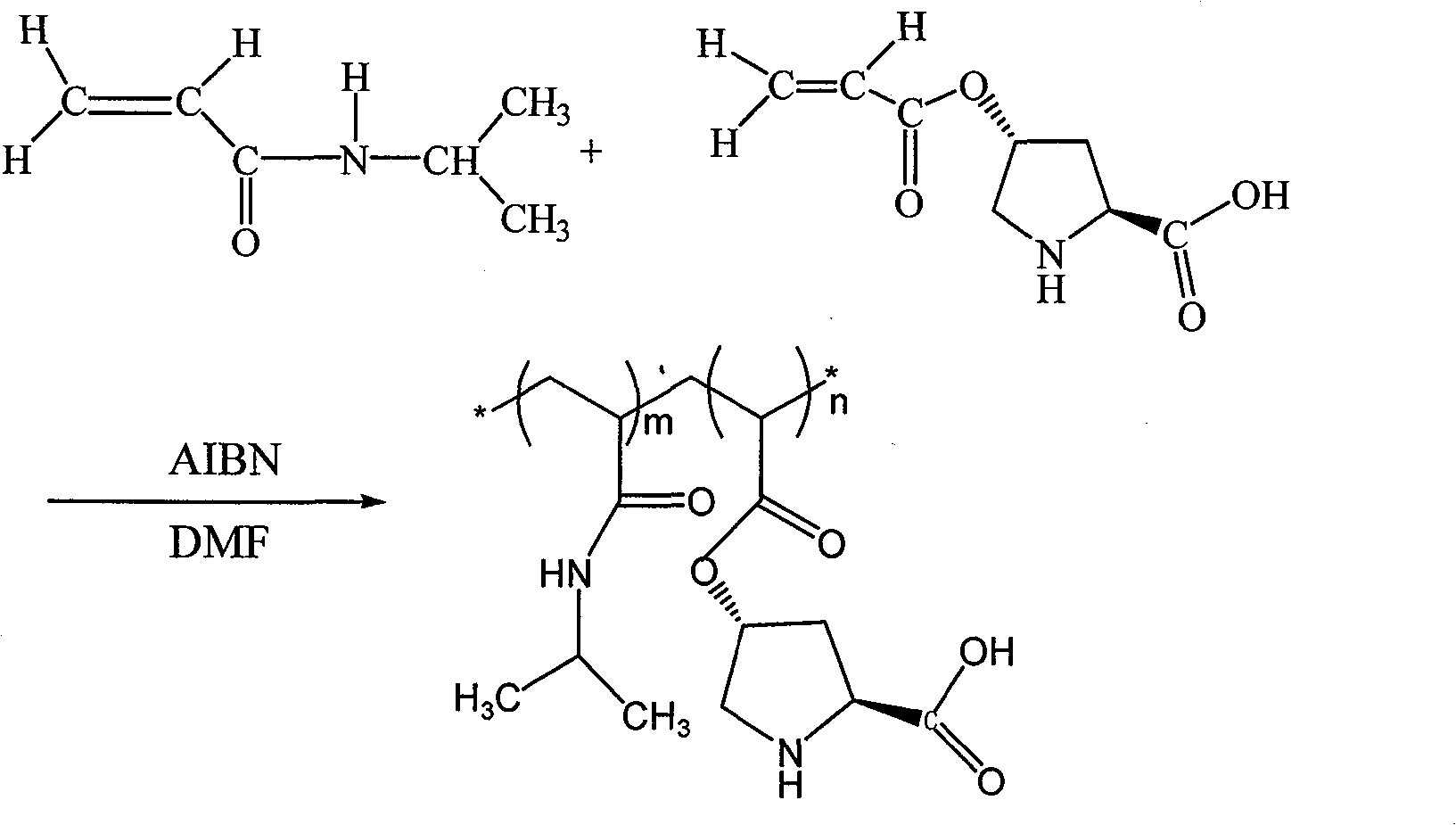
[0019] Take 2.54g of N-isopropylacrylamide and 0.56g of (s)-O-acryloyl-4-hydroxy-L-proline, 0.041g of initiator AIBN, and 15ml of solvent DMF into the reaction eggplant bottle in sequence, and stir Deoxygenate after dissolution. 80 DEG C of oil baths were reacted for 6 hours, after the end of the reaction, the product was precipitated with 120 ml of anhydrous ether as a precipitating agent, centrifuged, and the precipitate was placed in a vacuum oven for drying at 55 DEG C to obtain a catalyst, wherein m:n=9:1, ( 1 See HNMR figure 1 ).
Embodiment 2
[0021] A kind of preparation method of polymer chiral catalyst
[0022] Take 3.36g of N-isopropylacrylamide and 1.64g of (s)-O-acryloyl-4-hydroxy-L-proline, 0.061g of initiator AIBN, and 15m1 of solvent DMF into the reaction eggplant bottle in sequence, and stir Deoxygenate after dissolution. React in an oil bath at 80°C for 6 hours. After the reaction, use 120ml of anhydrous ether as a precipitant to precipitate the product, centrifuge, and dry the precipitate in a vacuum oven at 55°C to obtain a catalyst, wherein m:n=4:1.
Embodiment 3
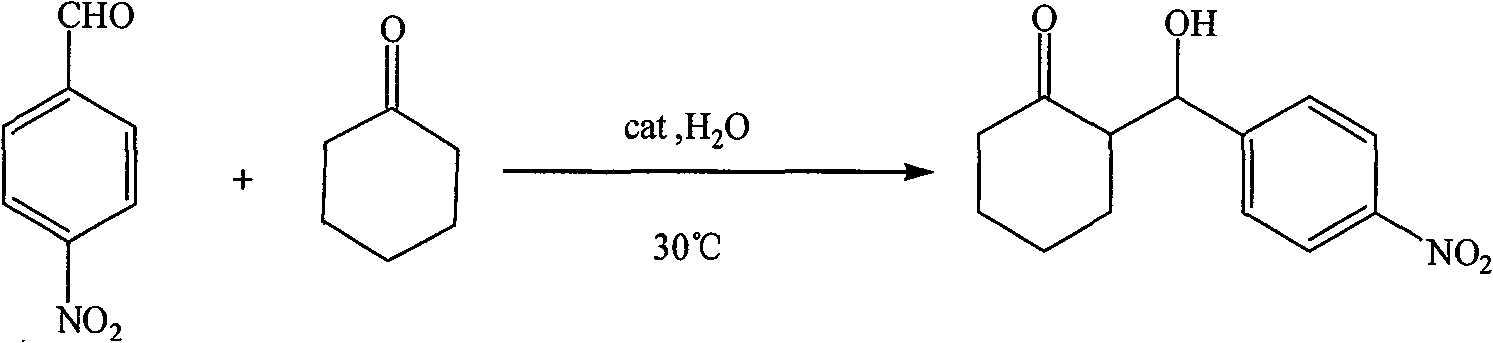
[0024] Take by weighing 0.5g prepared catalyst, 0.0756g 4-nitrobenzaldehyde, measure 2ml cyclohexanone, 2mlH 2 O, react at 30°C for 24h (see Table 1 for catalytic performance data). After the reaction is completed, the system is heated to 70° C., and the catalyst is recovered by centrifugation while it is hot.
[0025] The reaction formula is:
[0026]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com