Recombinant neutrophil gelatinase associated lipocalin and preparation method of protein antibody
A technology of lipocalin and neutrophils, which is applied in the field of biological science and carrier protein, can solve the problems of low expression yield, poor specificity and sensitivity, and insufficient polyclonal antibody, so as to increase the expression level and avoid poor specificity , the effect of sufficient amount of resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
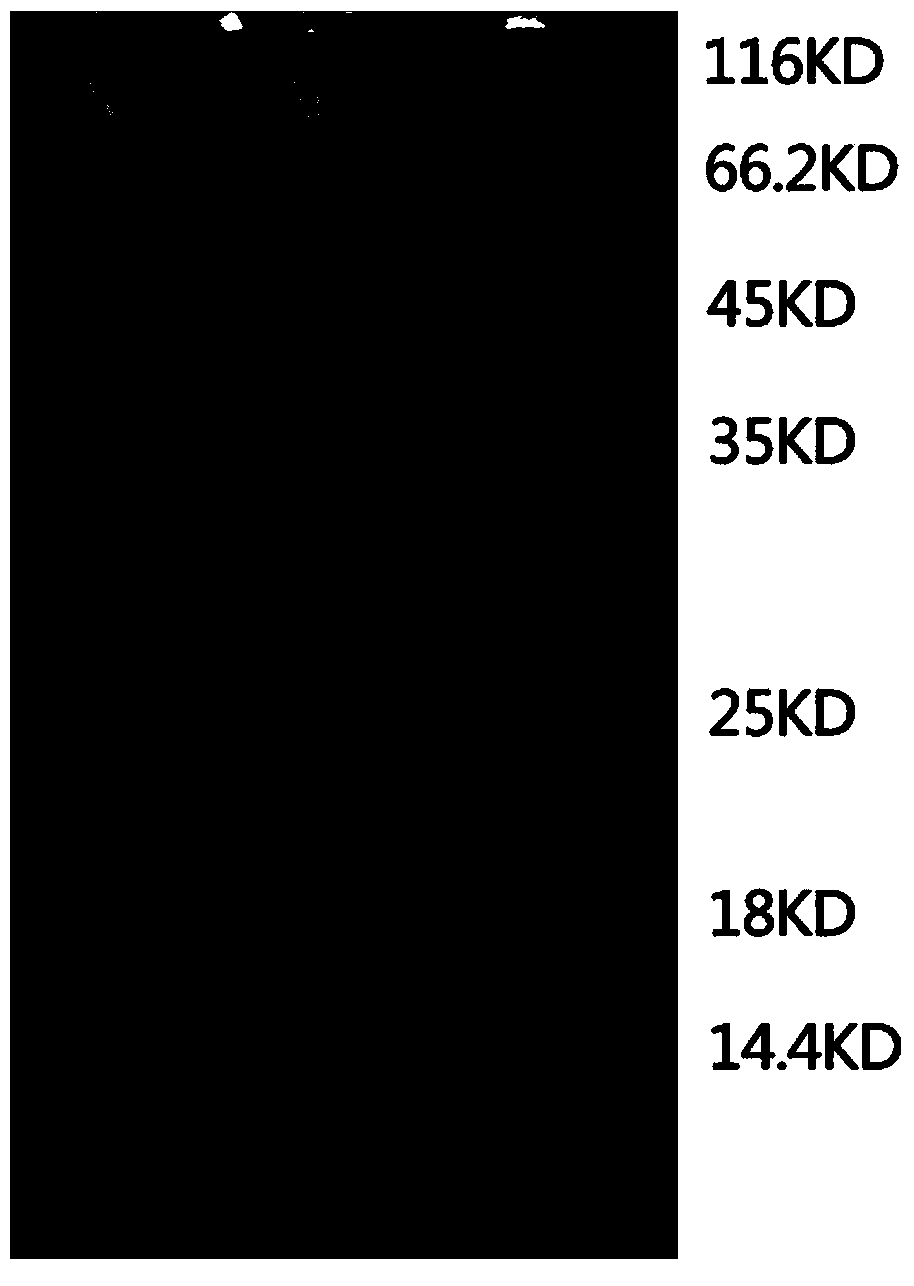
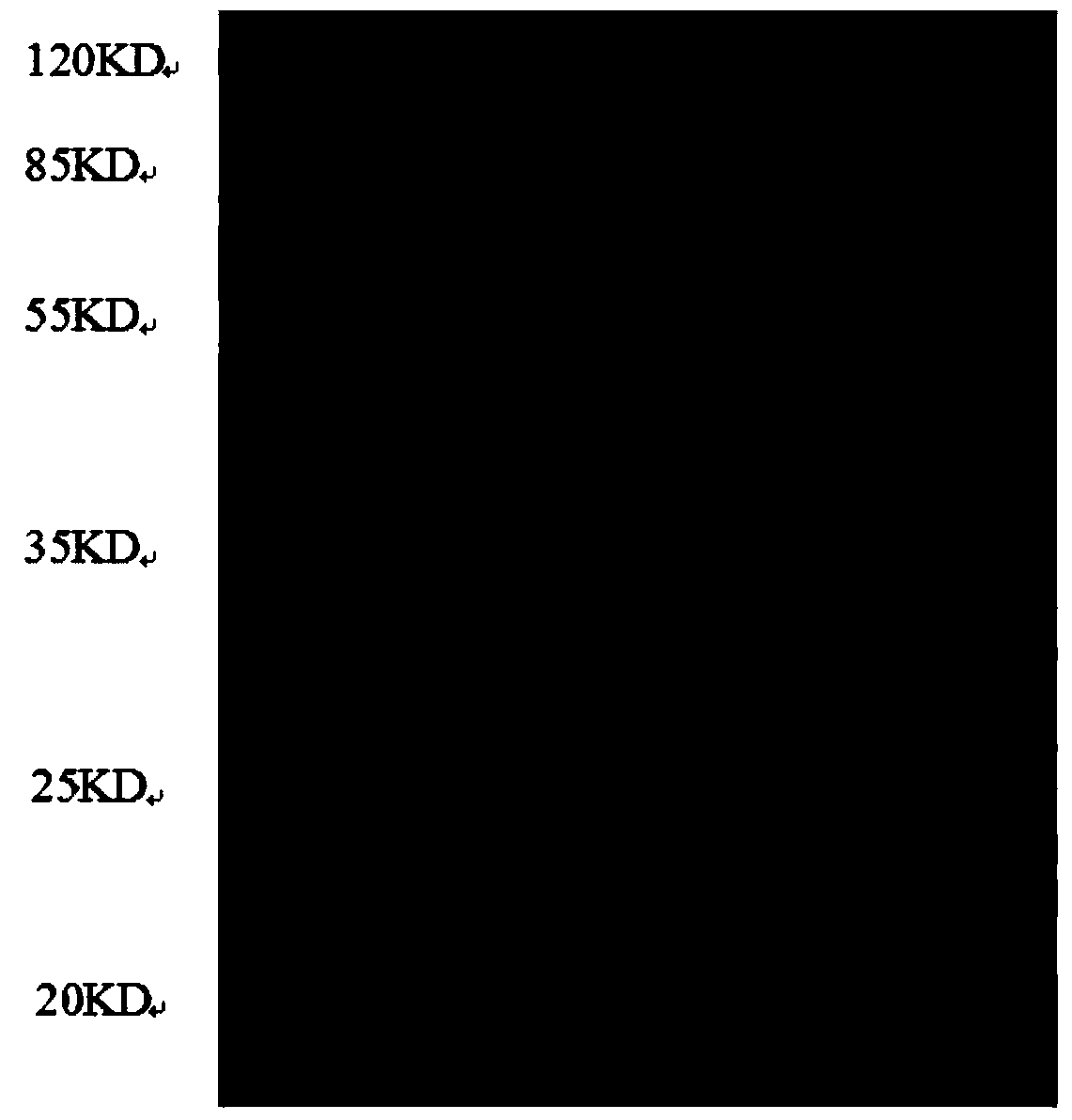
Image
Examples
Embodiment 1
[0030] 1. Expression of recombinant NGAL
[0031] 1. The sequence of SEQ ID NO.1 in the clone sequence table, the primers used are:
[0032] Upstream primer: 5'TACGGGATCCGCCCTGTTGGGGGCTCTGCATG3' (SEQ ID NO.2)
[0033] Downstream primer: 5'GCTAGTCGACGCCGTCGATACACTGGTCG3' (SEQ ID NO.3)
[0034] This primer pair was used to amplify the recombinant NGAL fragment sequence in SEQ ID NO.1 in vitro.
[0035] 2. Insert the amplified recombinant NGAL fragment in SEQ ID NO. 1 into the expression vector pFastBacTM Dual. First, digest the pFastBacTM Dual plasmid with BamHI and SalI to expose the cohesive ends that can be paired with the NGAL fragment, and then form a complete circular plasmid under the action of ligase. Then the circular plasmid was transformed into E. coli DH5α.
[0036] 3. Plasmid verification. After obtaining Amp-resistant transformants, 10 transformants were picked and cultured in LB medium containing 100 μg / mL Amp overnight, the plasmid was extracted, and the plasmid DNA was ...
Embodiment 2
[0052] This example is different from Example 1 in that the antibody preparation steps are different from Example 1. The antibody preparation steps are:
[0053] Antibody preparation
[0054] Immune goats with the protein obtained by separation and purification to prepare anti-recombinant NGAL polyclonal antibodies. The specific operation process is as follows: ① Male healthy young Boer goats, weighing more than 15KG, are randomly divided into groups, waiting to be immunized. One week before immunization, blood was collected from the jugular vein as a negative control; ②A certain amount of recombinant NGAL protein powder was prepared with sterile PBS buffer to make a solution with a concentration of 1 mg / mL and fully emulsified with the same amount of Freund’s complete adjuvant Inject the white rabbits prepared in advance for immunization, the injection volume is 0.6-0.8mg / head; ③Inject a booster injection three weeks after the first immunization injection, and then boost immuniza...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com