Electrochemical preparation method of graphene
An electrochemical and graphene technology, applied in the electrolysis process, electrolysis components, etc., can solve the problems of complicated and lengthy process, expensive equipment, and many graphene defects, and achieve high quality, high stripping efficiency and yield, and few defects. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0019] 20 mg of graphite powder was pressed into a graphite sheet (8 mm X 6 mm) by direct compression method, and then wrapped in a stainless steel mesh. Such as figure 1 In the diagram of the experimental device shown, the anode and cathode are self-made graphite sheet electrodes, the electrolyte is 1-methylimidazolium bisulfate, and a voltage of 4V is continuously applied for 5 hours. Transfer the cathode and anode expanded graphite into two agate mortars respectively, add 0.2ml of 1-methylimidazolium bisulfate, and grind for 3 hours. The gel-like mixture was transferred into a mixed solvent of acetone and DMF (V:V=2:1), and washed by high-speed centrifugation for 3 times to remove, the rotation speed was 13000rmp, and each time was 10min. The sediment was transferred into 50ml DMF, centrifuged at 2000rmp for 5min, and the supernatant was the obtained graphene solution, which was dark gray.
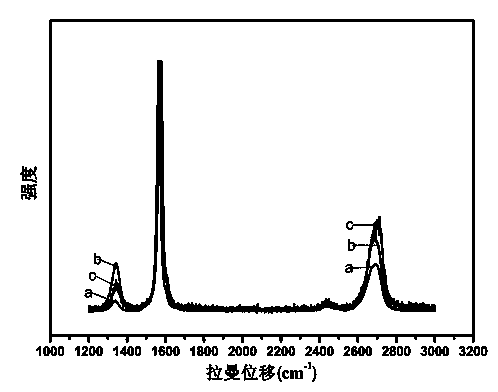
[0020] Such as figure 1 Raman diagram, Figure a is the graphene produced by the ...
example 2
[0022] Such as figure 1 In the diagram of the experimental device shown, both the anode and cathode are 20mg highly pyrolytic graphite sheets, the electrolyte is 1-butyl-3-methylimidazolium bisulfate, and a voltage of 5v is continuously applied for 5 hours. Transfer the cathode and anode expanded graphite into two agate mortars respectively, add 0.3ml of 1-butyl-3-methylimidazolium bisulfate, and grind for 3 hours. The gel-like mixture was transferred into a mixed solvent of acetone and DMF (V:V=1.5:1), and washed by high-speed centrifugation for 3 times to remove, the rotation speed was 13000rmp, and each time was 10min. The sediment was transferred into 50ml DMF, centrifuged at 5000rmp for 5min, and the supernatant was the prepared graphene solution, which was gray.
example 3
[0024] 20 mg of graphite powder was pressed into a graphite sheet (8 mm X 6 mm) by direct compression method, and then wrapped in a stainless steel mesh. Such as figure 1 In the diagram of the experimental device shown, the cathode and anode are self-made graphite sheet electrodes, the electrolyte is 1-butyl-3-methylimidazolium dihydrogen phosphate, and a voltage of 6V is continuously applied for 4.5 hours. Transfer the cathode and anode expanded graphite into two agate mortars respectively, add 0.5ml of the above-mentioned 1-butyl-3-methylimidazolium dihydrogen phosphate, and grind for 6 hours. The gel-like mixture was transferred into a mixed solvent of acetone and DMF (V:V=2.5:1), and washed by high-speed centrifugation for 3 times to remove, the rotation speed was 13000rmp, and each time was 10min. The sediment was transferred into 50ml DMF, centrifuged at 2000rmp for 5min, and the supernatant was the prepared graphene solution, which was gray.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com