Method for controlling dichloromethane residue in polymer microsphere preparation
A dichloromethane and polymer technology, which is applied in the field of controlling dichloromethane residues in polymer microsphere preparations, can solve problems such as increasing the dosage of administration, increase in particle size, and release effects, and achieves control of residual problems and high levels of production. The effect of drug loading and encapsulation efficiency, process stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] prescription:
[0025] Risperidone 3g
[0026] PLGA 75255A 3g
[0027] Dichloromethane 15ml
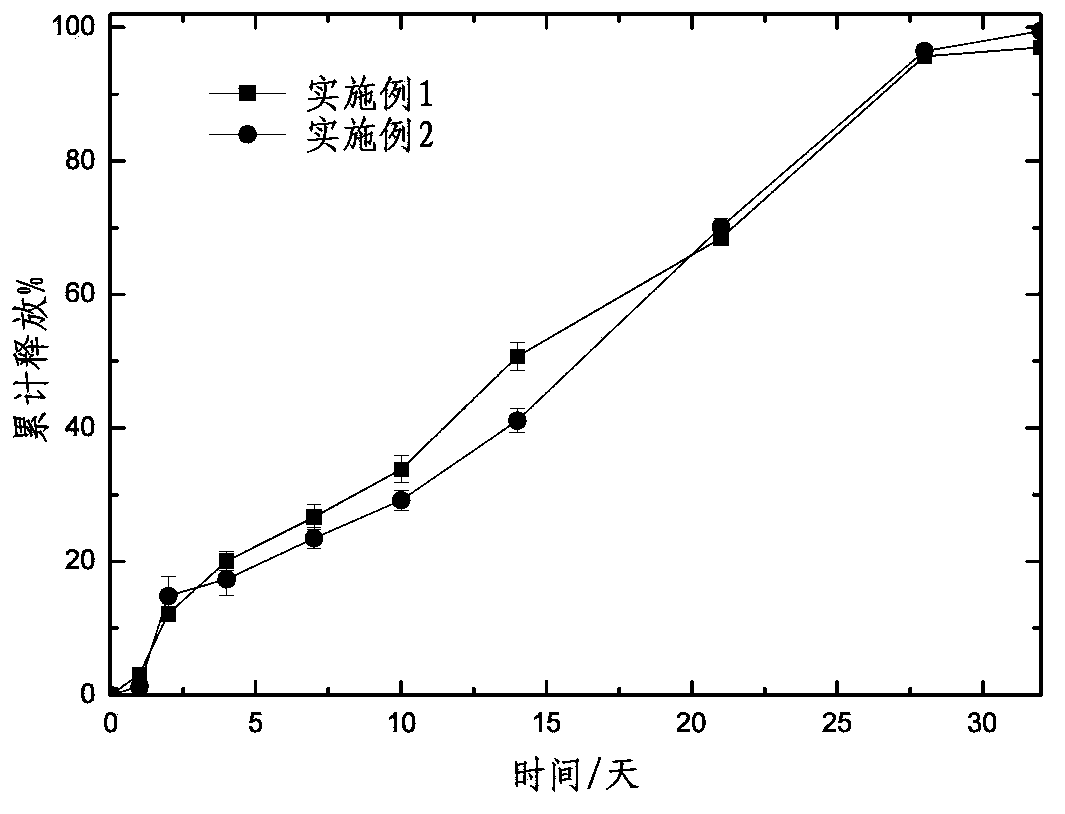
[0028] Preparation process: add 3g of PLGA 75255A and 3g of risperidone into 15ml of dichloromethane at room temperature, after being completely dissolved, add into 3L of water phase containing 1% PVA and emulsify and stir for 4min with a high-shear emulsifying disperser (rotating speed 900rpm), then stirred slowly at 100rpm for 4h, evaporated dichloromethane, filtered, collected and washed the microspheres with 10% ethanol, and freeze-dried at 45°C. After lyophilization, the appearance, content, encapsulation efficiency, release, etc. were determined according to the quality evaluation of the microsphere preparation.
Embodiment 2
[0030] prescription:
[0031]
[0032] Preparation process: Dissolve 3g of PLGA 75255A and 3g of risperidone in 15ml of dichloromethane at room temperature, add 3ml of benzyl alcohol, mix well and add to 3L of water phase containing 1% PVA with a high-shear emulsifying disperser Emulsify and stir for 4 minutes (rotating speed 900 rpm), then slowly stir at 100 rpm for 4 hours, evaporate dichloromethane, filter, collect and wash the microspheres with 10% ethanol, and freeze-dry at 45°C (lyophilization conditions are the same as Example 1). After lyophilization, the appearance, content, encapsulation efficiency, release, etc. were determined according to the quality evaluation of the microsphere preparation.
Embodiment 3
[0034] prescription:
[0035]
[0036]
[0037] Preparation process: Dissolve 3g PLGA 75255A in 15ml dichloromethane at room temperature, dissolve 2g risperidone in 9ml benzyl alcohol, mix well after completely dissolving, add to 3L water phase containing 1% PVA and 5% benzyl alcohol for use A high-shear emulsifying disperser was used to emulsify and stir for 4 minutes (rotating speed 900 rpm), followed by slow stirring at 100 rpm for 4 hours, dichloromethane was evaporated, filtered, collected and washed with 5% benzyl alcohol, and freeze-dried at 30°C. After lyophilization, the appearance, content, encapsulation efficiency, release, etc. were determined according to the quality evaluation of the microsphere preparation.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com