Preparation and application of human serum albumin-ruthenium inorganic drug complex
A human serum albumin, inorganic drug technology, applied in the field of anti-tumor drugs, to achieve the effect of improving targeting, high vascular permeability and retention rate, and reducing toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
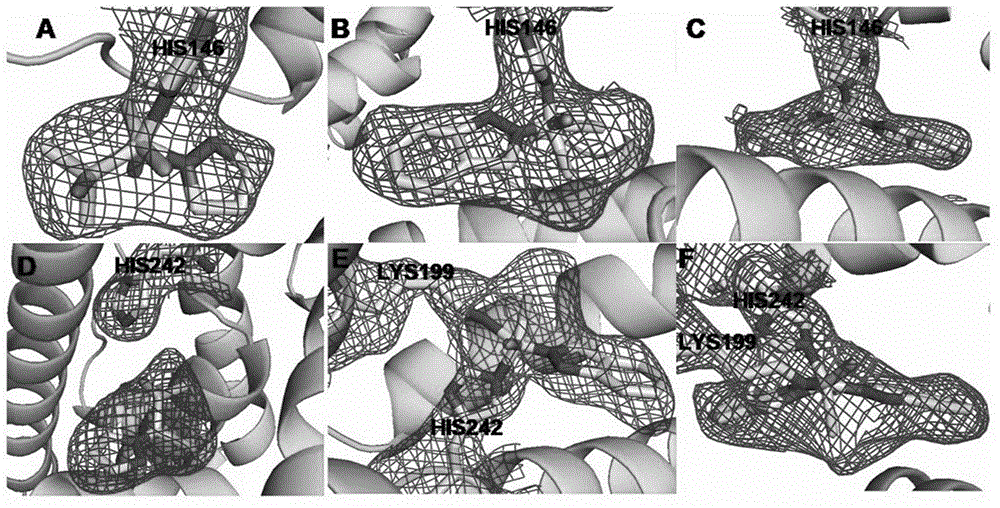
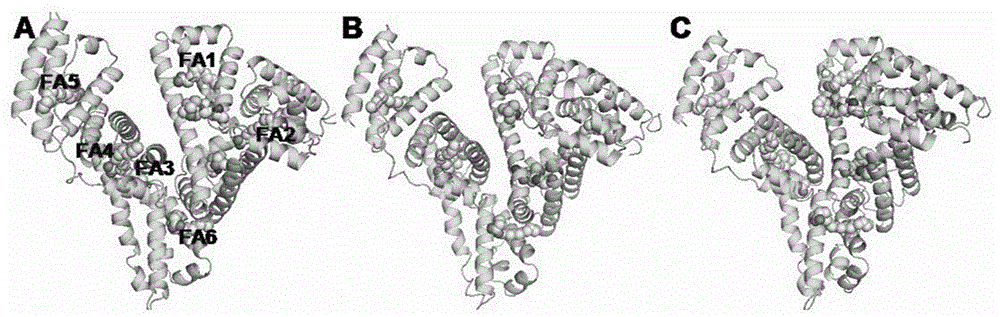
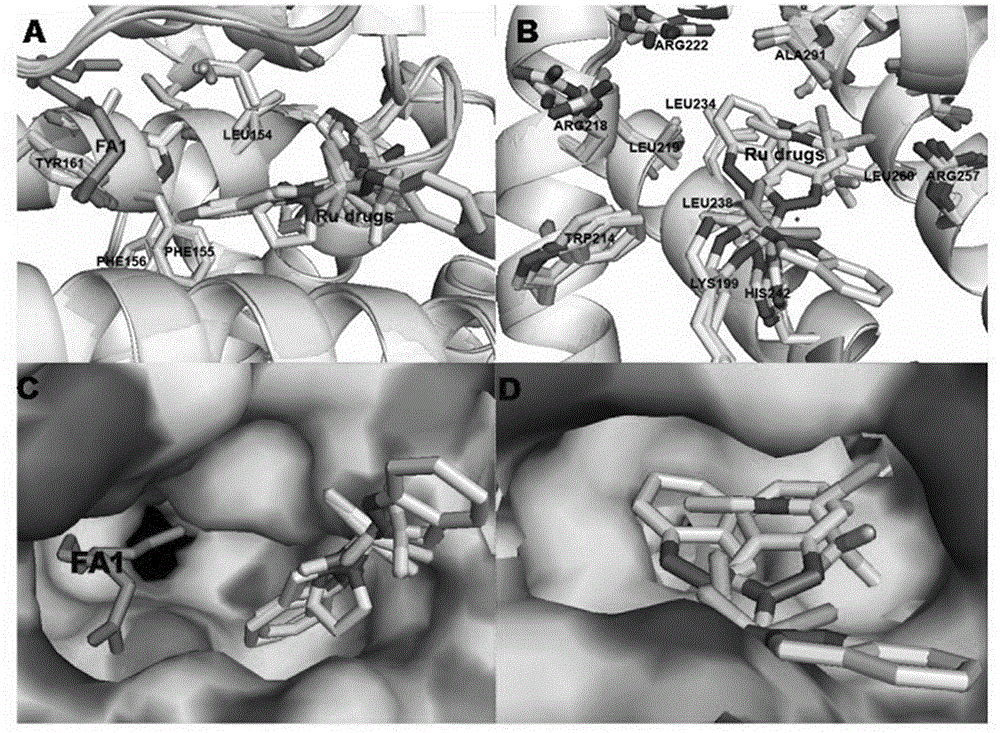
[0024] Embodiment 1: the preparation of human serum albumin-NAMI-A ruthenium inorganic drug complex
[0025] NAMI-A was prepared according to the literature: 1g RuCl 3 Add to 30ml of ethanol, reflux for 3 hours to obtain a dark green solution, filter, concentrate to one-tenth of the initial volume, then add 1ml of concentrated hydrochloric acid and 2ml of DMSO, keep at 80°C for 15 minutes to obtain a bright orange solution. Cool the solution to room temperature, add 10ml of acetone to obtain orange-red crystals, add a few drops of diethyl ether to accelerate its crystallization, and collect by filtration. Wash with 20ml of cold acetone, then wash with 10ml of diethyl ether, and dry in vacuo to obtain 1.5g with a yield of 72%. Suspend 1.0 g of the above product in 20 ml of acetone, add 0.49 g of imidazole, stir for 4 hours, the color changes from orange to brick red, wash with 10 ml of acetone and 10 ml of ether, and dry the product at 60°C. Weigh the prepared NAMI-A drug, c...
Embodiment 2
[0026] Embodiment 2: the preparation of human serum albumin-Ru (ind) ruthenium inorganic drug complex
[0027] Ru(ind) was prepared according to literature: at 60-70°C, 0.2g, 1.7mmol of indazole was dissolved in 1ml of 2N HCl, 2ml of Ru(Ⅲ) solution was added to the hot solution, and a reddish-brown solid appeared immediately. Filtration, washing with ethanol and ether, and vacuum drying yielded 1.82 g of the product, with a yield of 50%. The prepared RIC drug was weighed and prepared into a 100mM solution with DMSO, and the human serum albumin (HSA) was prepared into a 1.5mM solution with secondary deionized water. The DMSO solution of RIC and the aqueous solution of HSA were mixed at a ratio of 1:1, and the content of DMSO was not more than 5%, and incubated at 4°C for 24 hours. Concentrate the mixture of HSA and ruthenium inorganic anticancer drugs, wash it repeatedly with deionized water twice to make the content of DMSO not more than 0.01%, and then concentrate to the req...
Embodiment 3
[0028] Embodiment 3: Preparation of human serum albumin-KP1019 ruthenium inorganic drug complex
[0029] KP1019 was prepared according to the literature: at 70°C, indazole (0.6g; 5.1mmol) was dissolved in 8ml of 12N HCl, 2ml of Ru(Ⅲ) solution was added to the hot solution, stirred at 80-90°C for 15 minutes, and immediately appeared An ocher-colored substance appeared, continued stirring to remove HCl residues, filtered, washed with ethanol and ether, and dried in vacuo to obtain 0.296 g of the product, with a yield of 87%. The prepared NAMI-A drug was weighed and made into a 400mM solution with DMSO, and human serum albumin (HSA) was made into a 1.5mM solution with secondary deionized water. The prepared KP1019 drug was weighed and made into a 100mM solution with DMSO, and human serum albumin (HSA) was made into a 1.5mM solution with secondary deionized water. Mix the DMSO solution of KP1019 and the HSA aqueous solution at a ratio of 1:1, the content of DMSO should not exceed...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com