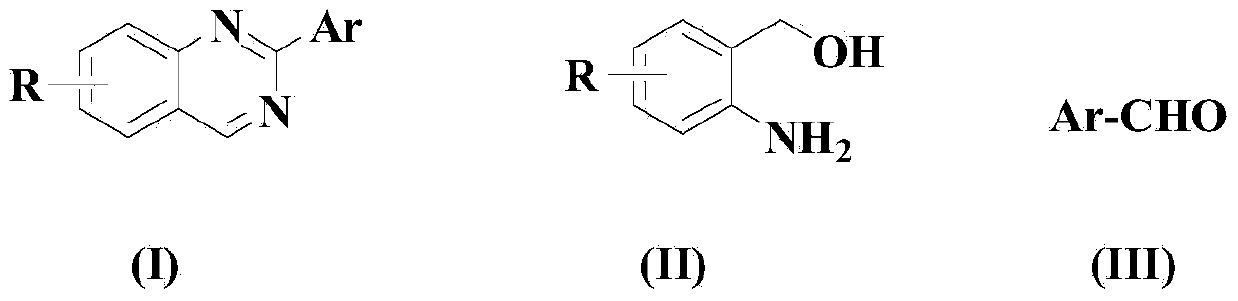
Method for synthesizing aryl or heteroaryl substituted quinazoline compound
A synthesis method and compound technology, which is applied in the synthesis of aryl or heteroaryl substituted quinazoline compounds and the synthesis of nitrogen-containing condensed ring compounds, and can solve the problems of high price, difficult synthesis of o-aminobenzylamine, and rare raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072] Embodiment 1: the synthesis of 2-phenylquinazoline
[0073]
[0074] Dissolve the compound of formula (II) in 100ml solvent acetonitrile, then add the compound of formula (III), after stirring evenly, add CuCl, FeCl in sequence 3 , ammonium chloride, KOH, 2,2'-bipyridine and TEMPO, so that (II):(III):CuCl:FeCl 3 : Ammonium chloride: KOH: 2,2'-bipyridine: TEMPO is 1:1:0.05:0.05:1:1:0.05:0.05 in molar ratio, wherein the compound of formula (II) is 10mmol.
[0075] In an air atmosphere, the above reaction system was reacted for 30 hours under stirring at 50°C. After the reaction, the solvent was removed from the mixture obtained after the reaction with a rotary evaporator, and the residue was purified by 200-300 mesh silica gel column chromatography to obtain the target product as a solid, with a yield of 92.8% and a purity of 99.1%. (HPLC).
[0076] Melting point: 97-98°C.
[0077] NMR: 1 H NMR (DMSO-d 6 ,500MHz)δ9.71(s,1H),8.56-8.59(m,2H),8.17(d,J=8.0Hz,1H),8.01...
Embodiment 2
[0079] Embodiment 2: the synthesis of 2-(4-fluorophenyl) quinazoline
[0080]
[0081] Dissolve the compound of formula (II) in 100ml solvent THF, then add the compound of formula (III), stir well, then add CuCl, FeBr in sequence 3 , ammonium sulfate, KOH, 4,4'-bipyridine and TEMPO, so that (II):(III):CuCl:FeBr 3 : Ammonium sulfate: KOH: 4,4'-bipyridine: TEMPO is 1:1.5:0.1:0.1:2:2:0.1:0.1 in molar ratio, wherein the compound of formula (II) is 10mmol.
[0082] In an oxygen atmosphere, the above reaction system was reacted for 25 hours under stirring at 60°C. After the reaction, the solvent was removed from the mixture obtained after the reaction with a rotary evaporator, and the residue was purified by 300-400 mesh silica gel column chromatography to obtain the target product as a solid, with a yield of 89.4% and a purity of 99.2%. (HPLC).
[0083] Melting point: 135-137°C.
[0084] NMR: 1 H NMR (CDCl 3,500MHz)δ9.43(s,1H),8.60-8.64(m,2H),8.06(d,J=8.3Hz,1H),7.89(t,J=8....
Embodiment 3
[0086] Embodiment 3: the synthesis of 2-(2-tolyl) quinazoline
[0087]
[0088] Dissolve the compound of formula (II) in 100ml solvent DMF, then add the compound of formula (III), stir well, then add CuCl, FeCl in sequence 3 , ammonium acetate, KOH, 2,2'-bipyridine and TEMPO, so that (II):(III):CuCl:FeCl 3 : Ammonium acetate: KOH: 2,2'-bipyridine: TEMPO is 1:2:0.2:0.2:3:3:0.15:0.15 in molar ratio, wherein the compound of formula (II) is 10mmol.
[0089] In an air atmosphere, the above reaction system was reacted for 20 hours under stirring at 70°C. After the reaction ended, the solvent was removed from the mixture obtained after the reaction with a rotary evaporator, and the residue was purified by 400-500 mesh silica gel column chromatography to obtain the target product as a viscous oil, with a yield of 84.3%, a purity of 98.8% (HPLC).
[0090] NMR: 1 H NMR (CDCl 3 ,500MHz)δ9.50(s,1H),8.10(d,J=8.3Hz,1H),7.89-7.94(m,2H),7.77(d,J=7.6Hz,1H),7.63(t,J =7.6Hz,1H),7.45(t,J...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com