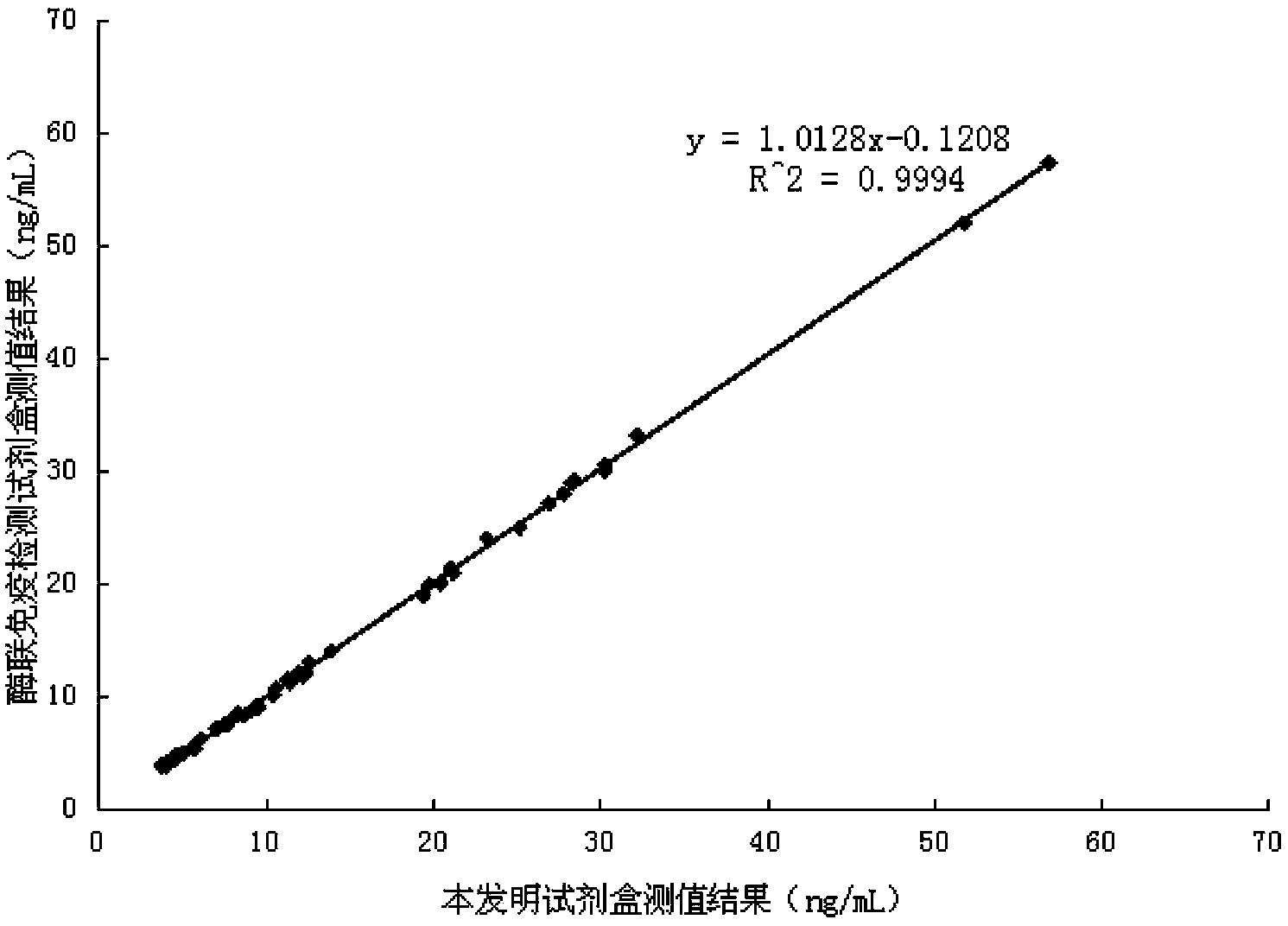
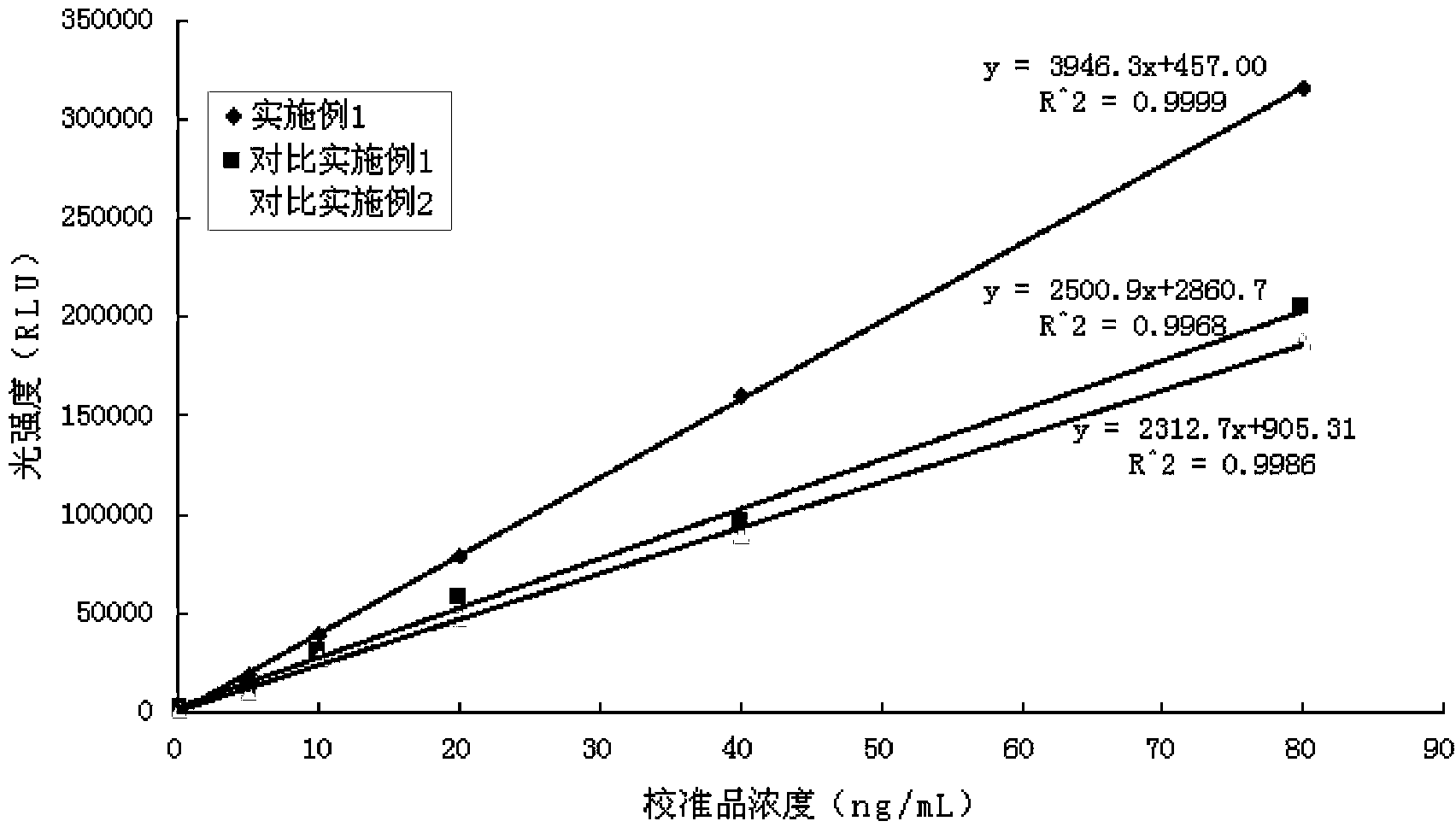
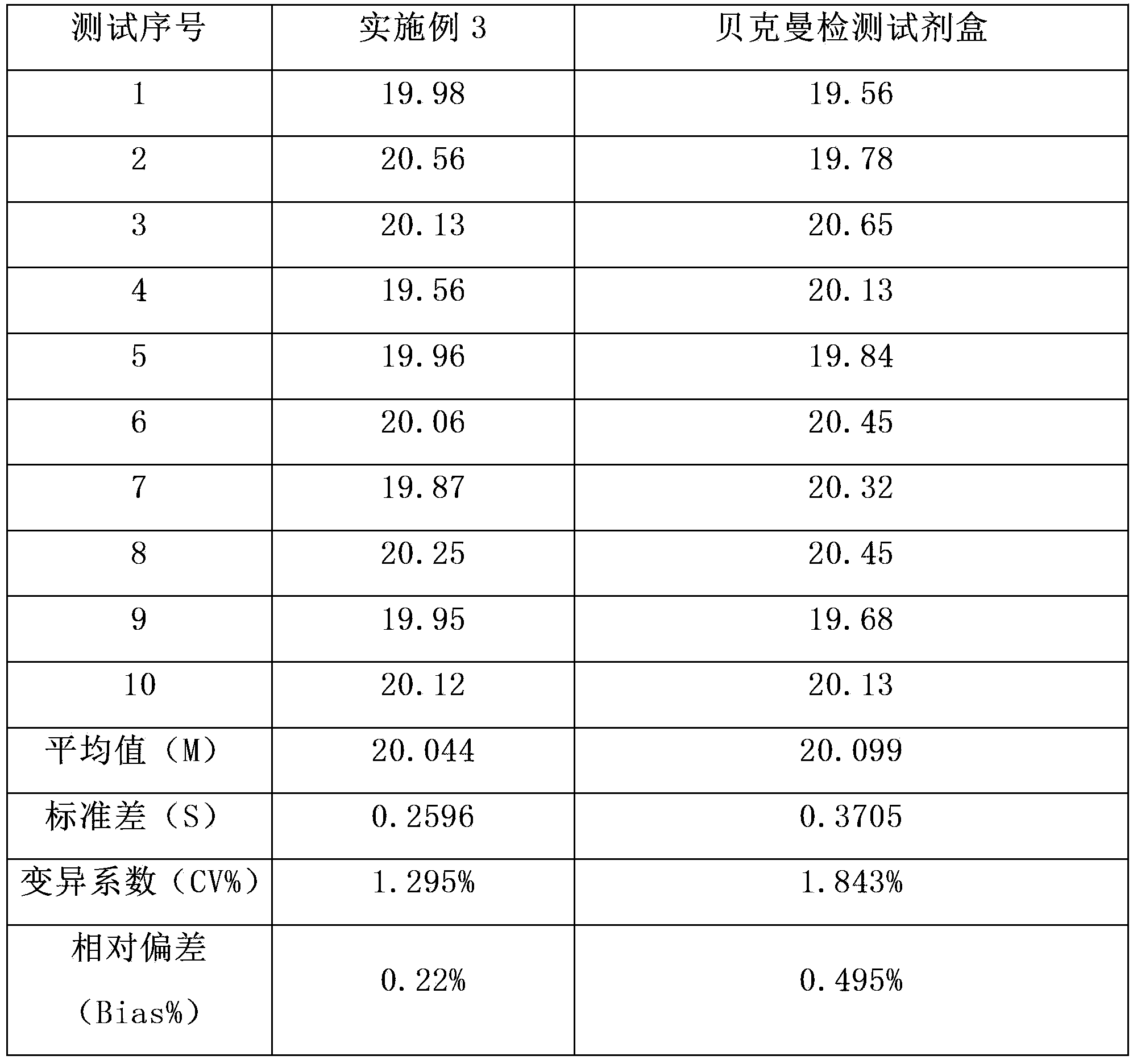
Magnetic particle chemiluminescence immune assay detection kit for carcino-embryonic antigen and detection method of detection kit
A chemiluminescence immunoassay, detection kit technology, applied in chemiluminescence/bioluminescence, analysis by chemical reaction of materials, analysis of materials, etc., can solve the problems of low degree of automation, narrow linear range, poor reproducibility, etc. Achieve the effect of overcoming technical defects and ensuring effectiveness and accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Preparation of detection kit in this embodiment:
[0043] 1. Preparation of carcinoembryonic antigen calibrator
[0044]①Preparation of hormone-free human serum: Take 20mL of normal human serum and put it in a centrifuge tube, then add 8.0g of activated carbon to the serum, put the centrifuge tube on a vortex mixer to mix evenly, oscillate for 5 hours, and centrifuge at 8000rpm for 30 minutes. Filter the supernatant, add sodium azide with a volume percentage concentration of 1 / 1000 to the filtrate, mix well and then freeze and store.
[0045] ② Take a certain amount of carcinoembryonic antigen protein (purchased from Bioss, USA) and use the above-mentioned hormone-free human serum to prepare a calibrator solution with a concentration of 80ng / mL, and dilute this calibrator with hormone-free human serum to obtain concentrations of 40ng / mL, 20ng / mL, 10ng / mL, 5ng / mL calibrator, aliquoted into aliquots with concentrations of 0ng / mL, 5ng / mL, 10ng / mL, 20ng / mL, 40ng / mL and 80n...
Embodiment 2
[0067] The difference between this example and Example 1 is that the magnetic beads used in the preparation process of anti-carcinoembryonic antigen monoclonal antibody-coated magnetic beads have a particle size of 1.0 μm (purchased from Germany Merck Company).
Embodiment 3
[0069] The difference between this example and Example 1 is that the magnetic beads used in the preparation process of the anti-carcinoembryonic antigen monoclonal antibody-coated magnetic beads have a particle size of 0.6 μm (purchased from Germany Merck Company).
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com