Method for preparing imatinib mesylate in alpha crystal form conveniently and rapidly
A technology of imatinib mesylate and crystal form, which is applied in the field of pharmaceutical chemical preparation, can solve the problems of expensive starting materials, complicated steps, complicated operations, etc., and achieve the effect of improving the utilization rate of atoms
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
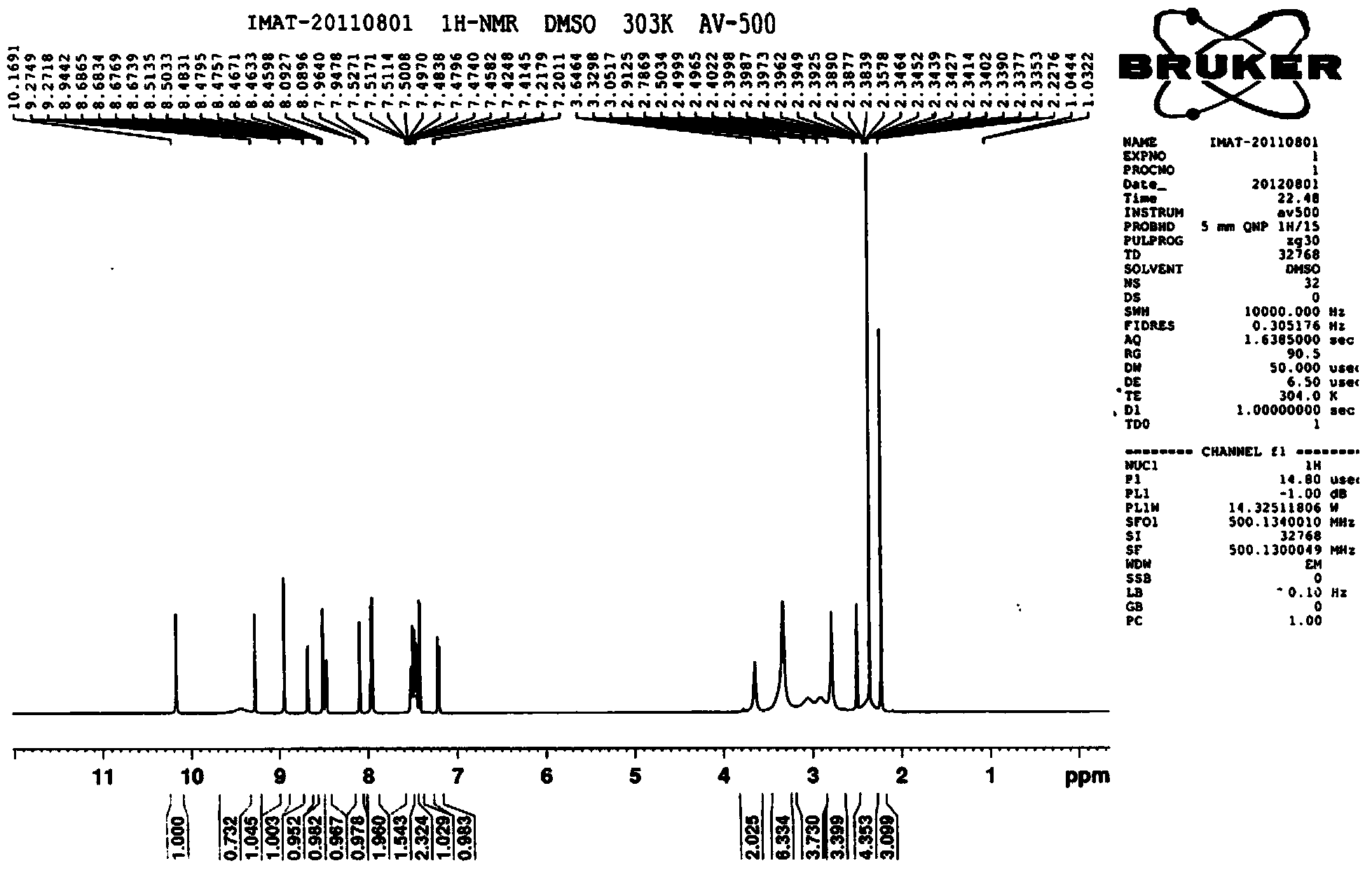
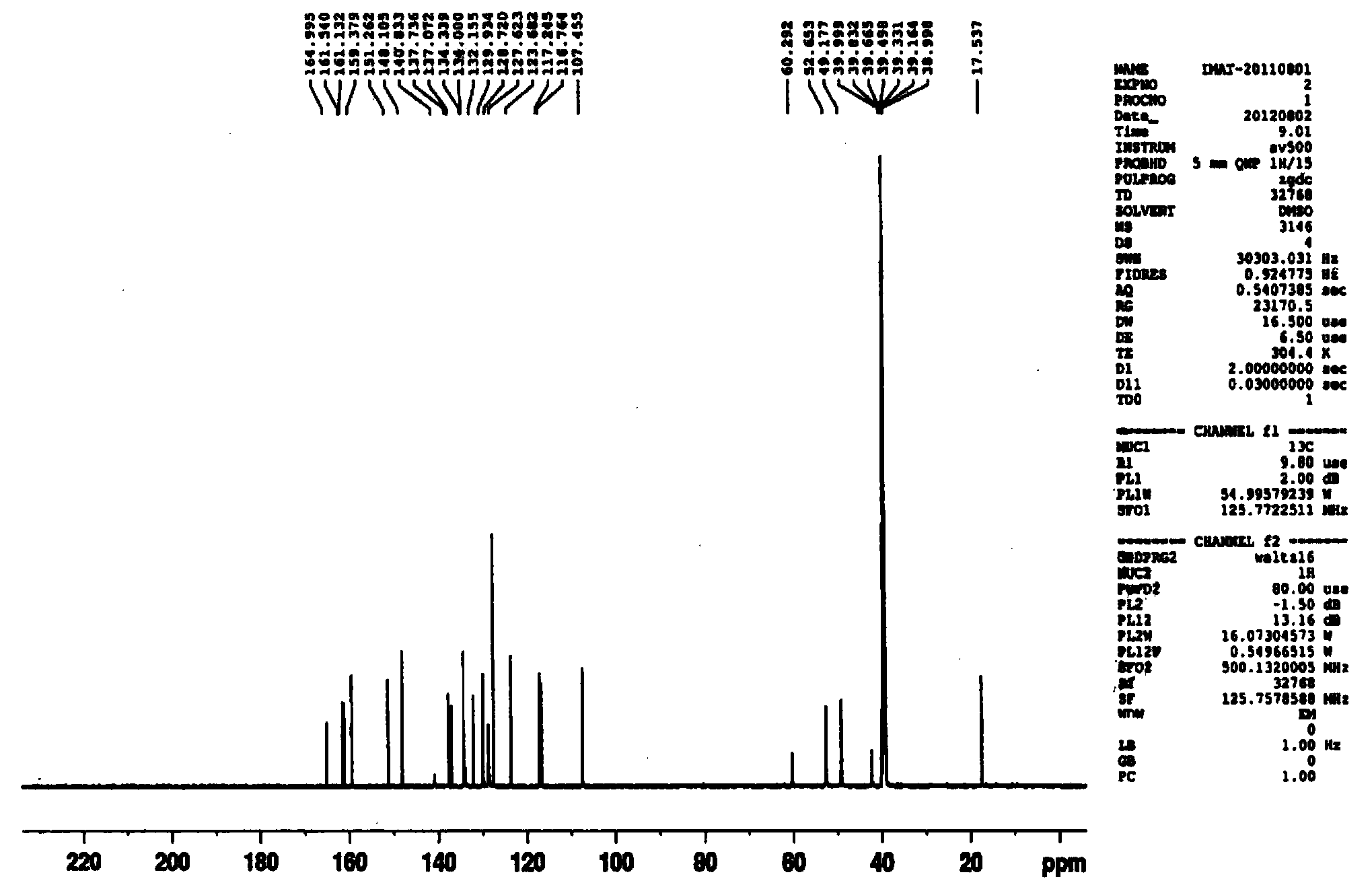
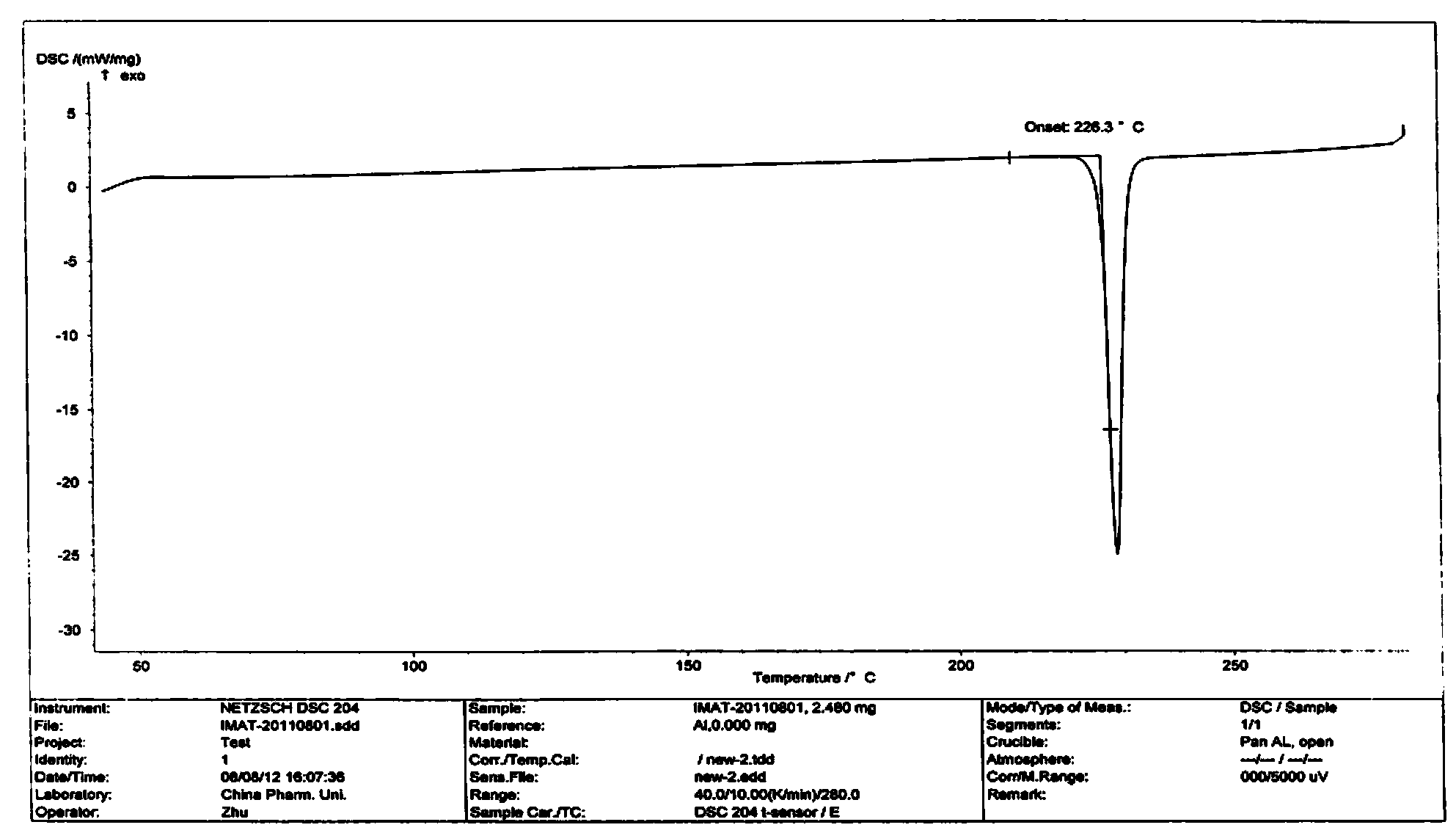
[0046] 4-(4-methylpiperazinemethyl) benzoic acid dihydrochloride (1Kg, 3.26mol) shown in formula II, triethylamine (0.992Kg, 9.8mol), add 1,4-dioxane 6L, stir evenly, cool down to 0°C, add methanesulfonyl chloride (0.375Kg, 3.26mol) dropwise, after dropping, slowly raise the temperature to 52°C, keep the temperature for 2-3h, and monitor the reaction by TLC to obtain a mixture of acids of formula II Anhydride solution, dimethylaminopyridine DMAP (41g, 0.33mol) was added. N-(5-amino-2-methylbenzene)-4-(3-pyridyl)-2-aminopyrimidine (0.904Kg, 3.26mol) represented by formula III was dissolved in 1.6L of dimethylformamide DMF , then add 2.4L of 1,4-dioxane, slowly drop the above amine solution into the above reaction solution, keep the temperature within 53-56°C, and control the dropping time within 1.5-2h. The reaction was incubated for 2.5 hours, and the reaction was complete as monitored by TLC. Slowly lower the temperature to 20°C, add 3L of ethyl acetate to the reaction solu...
Embodiment 2
[0050] Add 4-(4-methylpiperazinemethyl)benzoic acid dihydrochloride (1.5Kg, 4.88mol) and diisopropylethylamine (1.89Kg, 14.7mol) represented by formula II to 9.2L of acetonitrile, Stir evenly, cool down to 10°C, add methanesulfonyl chloride (0.559Kg, 4.88mol) dropwise, after dropping, slowly raise the temperature to 53-54°C, keep warm for 3 hours, monitor the reaction by TLC to obtain the mixed anhydride solution of the acid of formula II , and dimethylaminopyridine DMAP (61 g, 0.5 mol) was added. N-(5-amino-2-methylbenzene)-4-(3-pyridyl)-2-aminopyrimidine (1.35Kg, 4.88mol) represented by formula III was dissolved in 2.0L of dimethylformamide DMF , then add 3.1L of acetonitrile, slowly drop the above amine solution into the above reaction solution, keep the temperature within 53-54°C, control the dropping time within 1.5-2h, after dropping, keep warm for 2h, monitor the reaction by TLC completely. Slowly lower the temperature to 30°C, add 3.5L of ethyl acetate to the reactio...
Embodiment 3
[0054] 4-(4-Methylpiperazinemethyl)benzoic acid dihydrochloride (0.8 Kg, 2.60 mol) represented by formula II, N-methylmorpholine (0.790 Kg, 7.8 mol) were added to 4.4 L of tetrahydrofuran, Stir evenly, cool down to 2°C, add methanesulfonyl chloride (298g, 2.6mol) dropwise, after dropping, slowly raise the temperature to 54-56°C, keep the temperature for 3h, and monitor the reaction by TLC to obtain the mixed anhydride solution of the acid of formula II. Dimethylaminopyridine DMAP (35.5 g, 0.29 mol) was added. N-(5-amino-2-methylbenzene)-4-(3-pyridyl)-2-aminopyrimidine (720 g, 2.6 mol) represented by formula III was dissolved in 1.1 L of dimethylformamide DMF, Then add 1.8L of tetrahydrofuran, slowly drop the above amine solution into the above reaction solution, keep the temperature within 54-56°C, and control the dropping time within 1.5-2h. completely. Slowly lower the temperature to 25°C, add 2.8 L of ethyl acetate to the reaction solution, stir evenly, filter the precip...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com