Preparation methods and applications of benzylpenicilloic acid artificial antigen and benzylpenicilloic acid artificial antibody
A technology of penicillin thiazole and penicillin, which is applied in the field of preparation of benzyl penicillin artificial antigen and its antibody, achieving a high degree of similarity and retaining complete effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
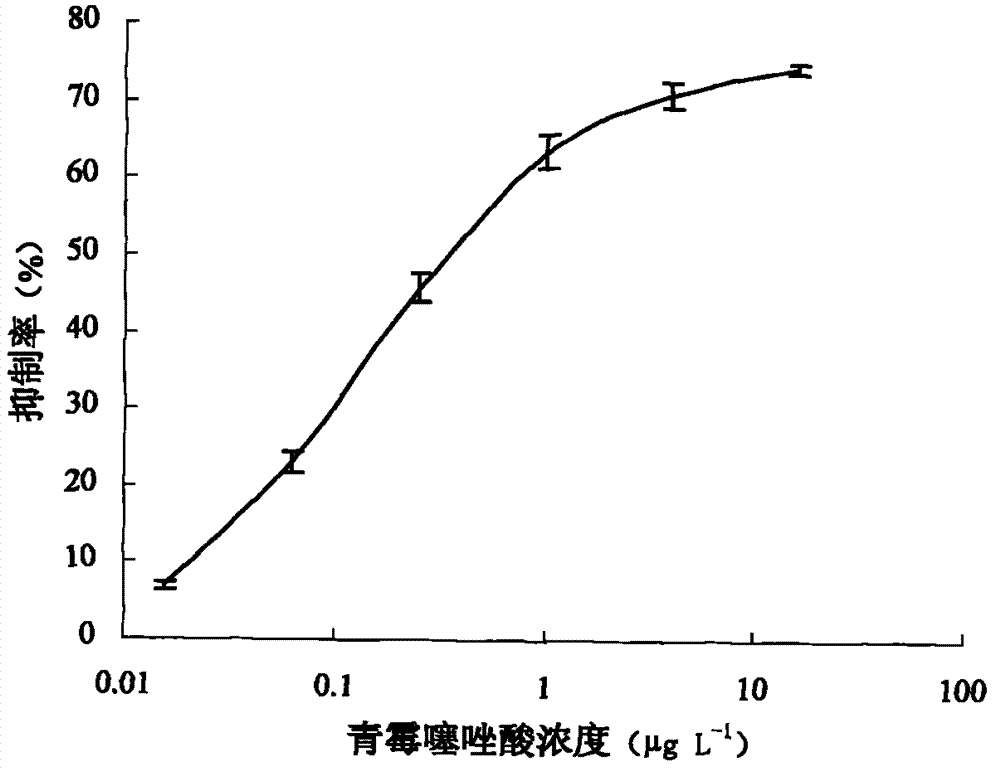
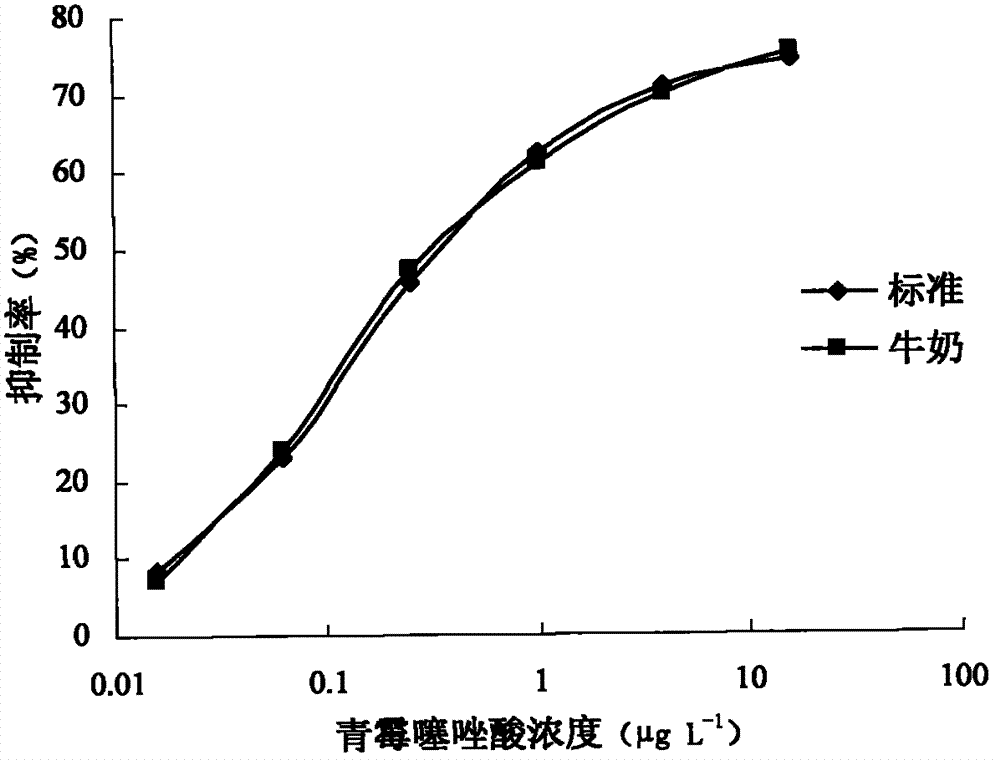
[0046] The preparation method of benzyl penicillin thiazole acid antibody:
[0047] The immunized animals were female New Zealand white rabbits. The immunization methods were subcutaneous and intramuscular injection. Four booster immunizations were carried out after the initial immunization. The booster immunizations were immunized three times after 2 weeks, 4 weeks and 6 weeks after the initial immunization respectively, and thereafter at intervals of one month. Carry out the fifth immunization, and 9 days later, blood is taken from the ear vein of the rabbit for titer detection. The specific method is as follows:
[0048] Initial immunization: take 1 mg of the artificial antigen synthesized according to the method of claim 3 and dissolve it in a solution prepared by equal volumes of 0.9% NaCl solution and Freund's complete adjuvant, and carry out animal immunization;
[0049] Booster immunization: immunize animals with a solution prepared by dissolving 0.5 mg of the above-me...
Embodiment 1
[0054] Preparation method of benzyl penicillin thiazole acid artificial antigen
[0055] With (2S, 5R, 6R)-3,3 dimethyl-6-(2-phenylacetamido)-7-oxo-4-thia-1-azabicyclo[3,2,0]heptane -2-Formic acid, with the amide group CO=NH as a bridge, is connected to hemocyanin (keyhole limpet hemocyanin, KLH) through the active ester method to synthesize artificial antigens; the specific method is:
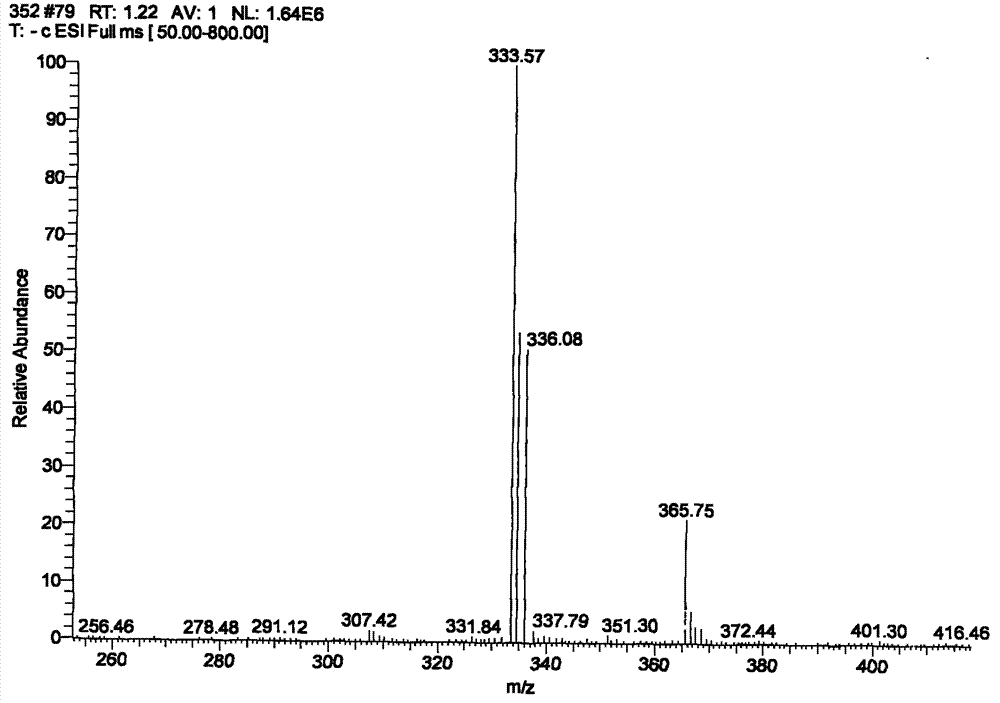
[0056] Take 1.5 mg of (2S, 5R, 6R) 3,3-dimethyl-6-(2-phenylacetamido)-7-oxo-4-thia-1-azabicyclo[3,2,0] Heptane-2-carboxylic acid potassium salt was added to 4.0 ml 1 M hydrochloric acid to obtain the hapten (2S,5R,6R)-3,3-dimethyl-6-(2-phenylacetamido)-7-oxo-4 - Thia-1-azabicyclo[3,2,0]heptane-2-carboxylic acid as a white solid, washed with double distilled water and dried to obtain the hapten.
[0057] Take 0.5000g of the obtained white solid and dissolve it in 2ml of THF, add 0.1500g of N-hydroxysuccinimide and 0.5000g of N,N-dicyclohexylcarbodiimide, and stir the reaction overnight at 22-...
Embodiment 2
[0059] Preparation method of penicillin thiazole acid antibody
[0060] The immunized animals were female New Zealand white rabbits. The immunization methods were subcutaneous and intramuscular injection. Four booster immunizations were carried out after the initial immunization. The booster immunizations were immunized three times after 2 weeks, 4 weeks and 6 weeks after the initial immunization respectively, and thereafter at intervals of one month. Carry out the fifth immunization, and 9 days later, blood is taken from the ear vein of the rabbit for titer detection. The specific method is as follows:
[0061] Initial immunization: take 1 mg of the artificial antigen synthesized according to the method of claim 3 and dissolve it in a solution prepared by equal volumes of 0.9% NaCl solution and Freund's complete adjuvant, and carry out animal immunization;
[0062] Booster immunization: immunize animals with a solution prepared by dissolving 0.5 mg of the above-mentioned arti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com