Graft materials derived from mammalian cartilage
A mammalian and cartilage technology, applied in animal cells, vertebrate cells, microorganisms, etc., can solve problems such as high cost, difficult to handle, and the spread of pathogens
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Determination of the optimal dose of embodiment 1.γ-ray
[0057] Fresh cartilage extracted from pig ears was soaked in 9% NaCl solution for 5 h and irradiated with ultrasound. Chondrocytes are removed by this treatment. Subsequently, it was immersed in 1% hydrogen peroxide as a virus inactivating solution for 45 minutes and stirred. Viruses and other pathogens are inactivated by this treatment. Then, it was immersed in 0.5M sodium hydroxide (NaOH) as an alkaline solution for 30 minutes. Before each treatment, the pre-treatment solution was thoroughly washed out with the washing solution. Dehydration was performed by sequential immersion in 50%, 80%, and 100% acetone to minimize damage to cartilage tissue, followed by sealing in containers and gamma-irradiation. The cartilage graft prepared by the above method was gamma-irradiated in units of 5 kGy in the range of 5 kGy to 30 kGy in order to determine the optimal dose of gamma rays. The optimal dose of γ-rays was ...
Embodiment 2
[0063] Example 2. Comparison of dehydration
[0064] The prepared cartilage was dehydrated by treatment with acetone or glycerol. Glycerol treatment is performed by soaking the tissue in glycerol including about 0.11% antibiotic and then soaking in greater than 99.9% glycerol to replace water in the tissue with glycerol.
[0065] refer to Figure 9 , comparing the thermal properties of the final product after dehydration by using glycerol. According to the change of thermal properties with dehydration shown in the figure Figure 9 , the glass transition temperature of acetone is about 99°, and that of glycerol is about 96°. Therefore, no significant difference was noted, and the thermal properties of acetone and glycerol did not affect the shape of the specimen. According to the change of cytotoxicity with dehydration according to the graph Figure 10 , also noted the absence of cytotoxicity using acetone or glycerol for dehydration.
[0066] Therefore, the cartilage g...
Embodiment 3
[0068] The mensuration of the ECM factor of embodiment 3. technology
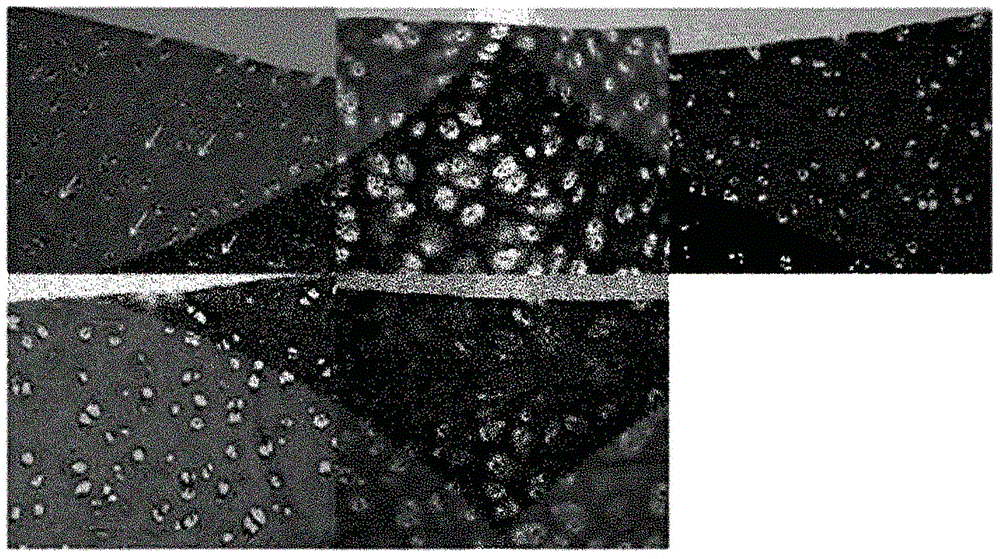
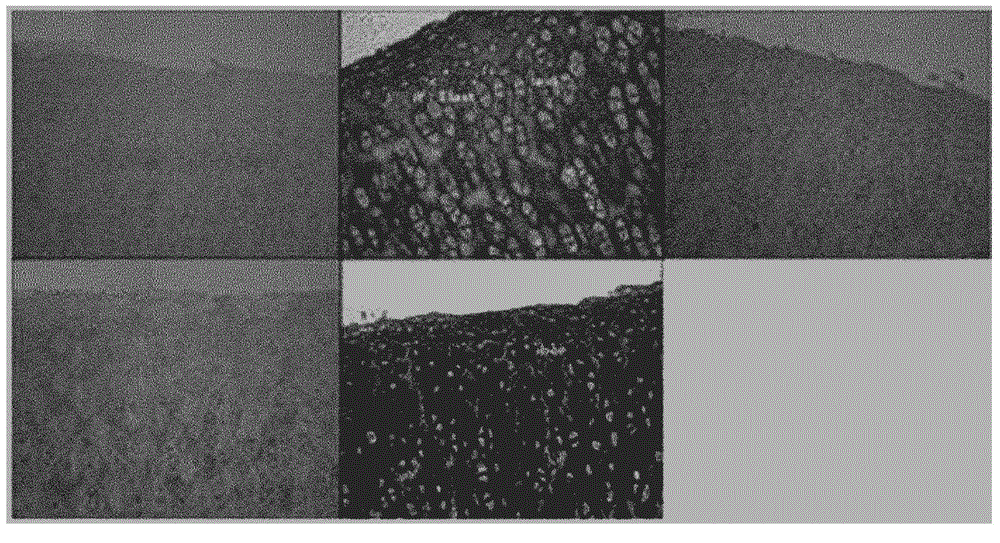
[0069] Such as Figure 5 and Figure 6 The amounts of keratan sulfate, collagen II, fibronectin, and chondroitin sulfate in fresh porcine ear cartilage and final cartilage grafts were determined as indicated in . Keratan sulfate was observed everywhere in the stroma, while chondroitin sulfate was mainly observed near the perichondrium, and chondroitin sulfate was especially concentrated on one side of the perichondrium. Note that the amount of Keratan Sulfate, Collagen II, Fibronectin and Chondroitin Sulfate in the final product decreased slightly, but remained and maintained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com