Spiro-containing dihydropyrazole compounds
A compound and cycloalkyl technology, applied in the field of dihydropyrazole compounds containing a spiro ring, can solve the problems of easy to cause hyperkalemia, poor selectivity, affecting clinical wide application, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
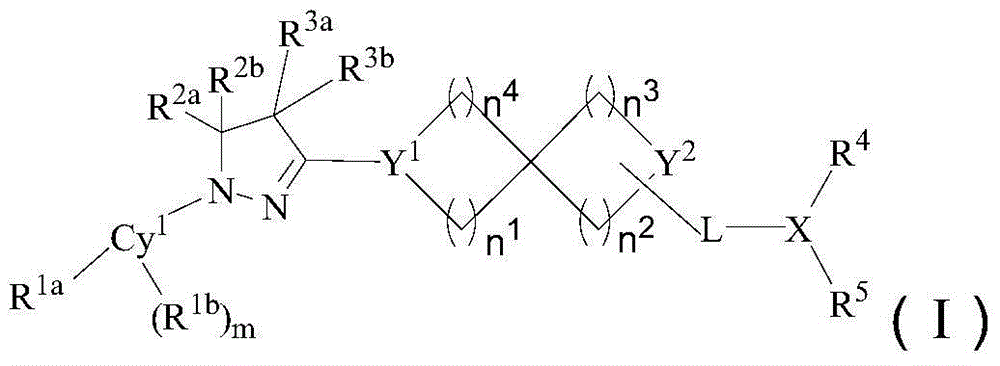
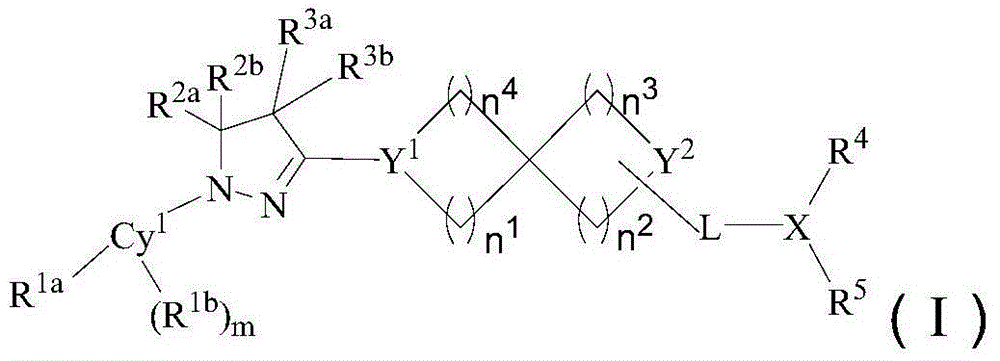
[0175] The preparation of formula (I) compound
[0176] Dissolve raw material 1 (1.3-2 equivalents) in a polar aprotic solvent (such as N,N-dimethylacetamide), add raw material 2 (1 equivalent), and finally add 3 equivalents of tertiary amine (including but not limited to diiso Propylethylamine), terminate the reaction after reacting at 90°C-120°C for 3-6 hours, pour into water after cooling, extract, dry the organic phase, spin dry, and purify with preparative liquid chromatography to obtain the compound of formula (I).
[0177] In the reaction equation, Cy 1 , L, X, Y 1 , Y 2 , n 1 , n 2 , n 3 , n 4 , R 1a , R 1b , R 2a , R 2b , R 3a , R 3b , R 4 , R 5 , and m are as defined above.
[0178] The pharmaceutical composition of the present invention containing the compound of general formula (I), its pharmaceutically acceptable salt, ester or solvate, or their prodrug or isomer may contain one or more pharmaceutically acceptable carriers.
[0179] The term "pharm...
specific Embodiment approach
[0211] The above-mentioned content of the present invention will be further described in detail through specific implementation in the form of examples below, but it should not be understood that the scope of the above-mentioned theme of the present invention is limited to the following examples.
[0212] In the examples, the raw material compounds used are commercially available, obtained from Shanghai Jingyan Chemical, Shanghai Titan Chemical, Shanghai Darui, Beijing Coup Technology Co., Ltd., Zhengzhou Taiji Hongnuo Pharmaceutical Technology Co., Ltd., Sichuan Guanghan Biology, Shaoyuan (Shanghai) Chemical Technology, Alfa Aisha (China), Shanghai TCI, Beijing Bailingwei, Shanghai Biide Pharmaceutical and other companies.
Embodiment 1
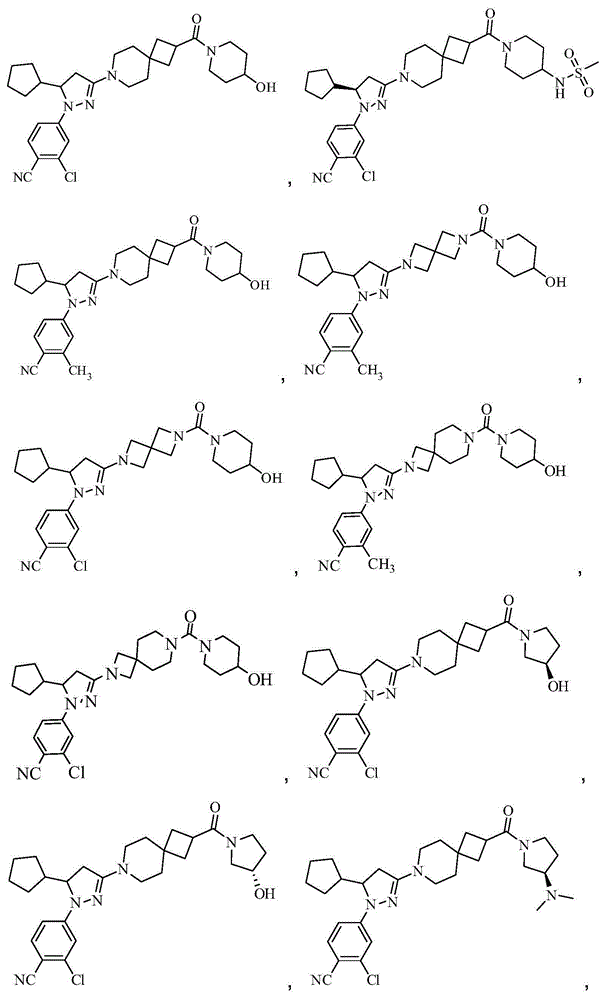
[0213] Example 12-Chloro-4-(5-cyclopentyl-3-(2-(4-hydroxypiperidine-1-carbonyl)-7-azaspiro [3.5] Preparation of nonan-7-yl)-4,5-dihydro-1H-pyrazol-1-yl)benzonitrile (compound 2)
[0214]
[0215] (1) Preparation of tert-butyl 2-(4-hydroxypiperidine-1-carbonyl)-7-azaspiro[3.5]nonane-7-carboxylate
[0216]
[0217] In a dry round bottom flask, add 7-(tert-butoxycarbonyl)-7-azaspiro[3.5]nonane-2-carboxylic acid (1.2g, 4.46mmol), 4-hydroxypiperidine (0.50g , 4.94mmol), DMF30mL, N,N-diisopropylethylamine (DIEA) (0.6g, 4.64mmol), and finally added 2-(7-azobenzotriazole)-N,N,N' , N'-tetramethyluronium hexafluorophosphate (HATU) (1.86g, 4.89mmol), react overnight at room temperature. The reaction solution was poured into 150 mL of ice water, extracted with dichloromethane (100 mL × 3), the organic phase was washed with a saturated solution of sodium bicarbonate, dried, and purified by column chromatography (petroleum ether: ethyl acetate = 1: 1) to obtain Light yellow visc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com