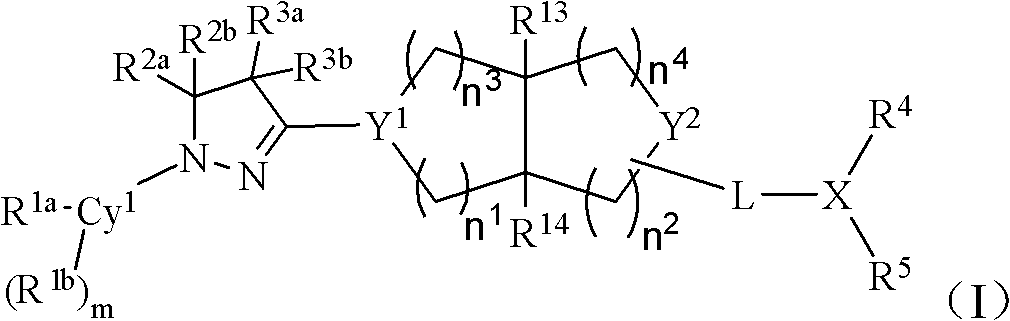
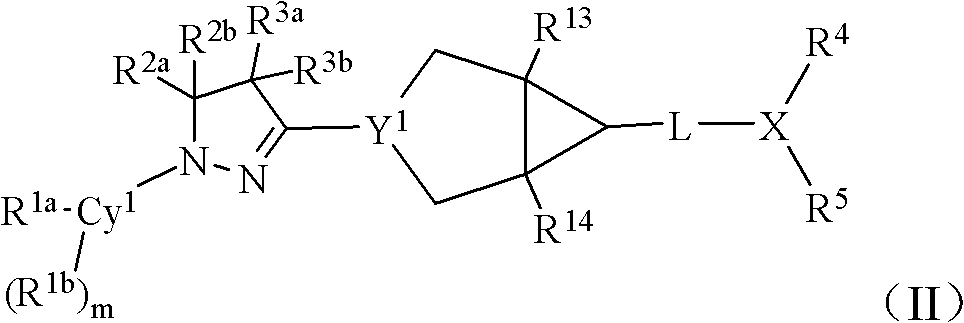
Bicyclic dihydropyrazole compounds
A compound and hydrogen atom technology, applied in the field of medicine, can solve problems such as large side effects, poor activity, and difficulty in synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0143] (1) Preparation of intermediate 1 hydrochloride
[0144] In a dry round-bottomed flask, add the derivative of raw material 1 (1 eq), raw material 2 (1.1 eq), DMF, DCM, DIEA (1.1 eq. ), and finally add HATU (1.1 equivalents), react overnight at room temperature. The reaction solution was poured into ice water, extracted with an organic solvent, the organic phase was washed with a saturated sodium bicarbonate solution, dried, and purified by column chromatography to obtain intermediate 1 with a protective group.
[0145] The above-mentioned intermediate 1 with a protective group is removed by a common method, for example, the intermediate 1 with a protective group is dissolved in dichloromethane, and dry HCl gas is introduced into the ice bath system for half an hour. After the reaction is completed, , spin to dry the solvent, and wash with ether to obtain the hydrochloride of Intermediate 1.
[0146] (2) preparation of formula 1 compound
[0147] The hydrochloride sal...
experiment example
[0175] Experimental example In vitro pharmacological activity of the compounds of the present invention
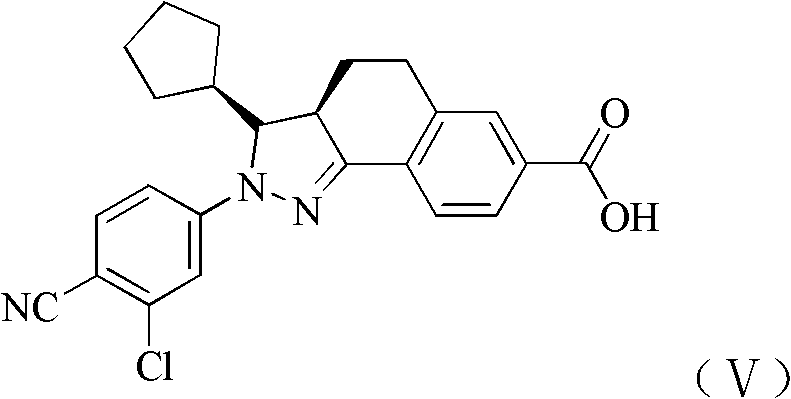
[0176] Test sample: some compounds 1, 3, 4, 6, 8, 9, 10, 11, 14, 15, 16, 17, 18, 19 and 27 of the present invention are self-made, and their chemical names and structural formulas are as described above.
[0177] The compound of formula V is self-made, and its structural formula is as described above.
[0178] Nuclear receptor assay
[0179] experimental method:
[0180] Accurately weigh the test compound 1 and 3, add DMSO to dissolve, mix thoroughly, and make 1000 μM. Then the above mother solution was serially diluted with DMSO to 200 μM, 40 μM, 8 μM, 1.6 μM, 0.3 μM, 0.06 μM, 0.01 μM, 0 μM, 0 μM.
[0181] Dual-luciferase detection: Mix 1 μL pBind-NR (100ng / μL), 1 μL pG5luc (100ng / ul), 2.5 μL DMEM and 0.5 μL Fugene, incubate at room temperature for 15 minutes, and prepare a transfection solution. According to 3×10 5 cells / mL Prepare a cell suspension, add 100 μL to ...
Embodiment 1
[0189] Example 1: 2-chloro-4-{5-cyclopentyl-3-[(1α, 5α, 6α)-6-(4-hydroxypiperidine-1-carbonyl)-3-azabicyclo[3.1. 0] hexane Preparation of -3-yl]-4,5-dihydro-1H-pyrazol-1-yl}benzonitrile (compound 1)
[0190]
[0191] (1) Preparation of (1α, 5α, 6α)-6-(4-hydroxypiperidine-1-carbonyl)-3-azabicyclo[3.1.0]hexane-3-carboxylic acid tert-butyl ester
[0192]
[0193] In a dry round bottom flask, add (1α, 5α, 6α)-3-tert-butoxycarbonyl-3-azabicyclo[3.1.0]hexane-6-carboxylic acid (1.50 g, 6.6 mmol) respectively, 4-Hydroxypiperidine (0.73g, 7.2mmol), DMF 30mL, CH 2 Cl 2 30 mL, DIEA (0.90 g, 7.0 mmol), finally added HATU (2.76 g, 7.3 mmol), and reacted at room temperature overnight. The reaction solution was poured into 250 mL of ice water, extracted with dichloromethane (100 mL × 3), the organic phase was washed with saturated sodium bicarbonate solution, dried, and purified by column chromatography (petroleum ether: ethyl acetate = 1: 1) to obtain Light yellow viscous liq...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com