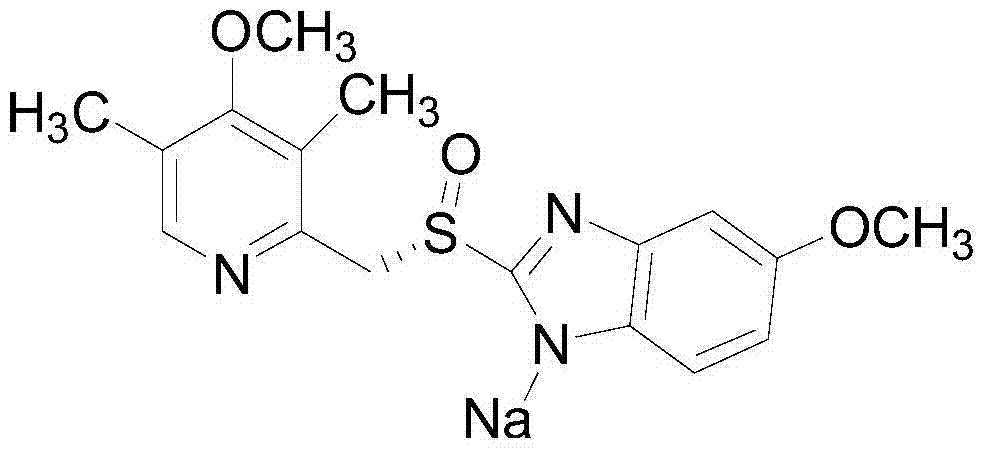
Esomeprazole sodium freeze-dried preparation and preparation method thereof
A technology for esomeprazole sodium and freeze-dried preparations, applied in the field of medicine, can solve problems such as blood calcium reduction, calcium ion reduction, personal safety impact, etc., and achieve the effects of reducing water content, not easy to shrink, and improving production efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038]
[0039] Specific steps:
[0040] Weigh each raw and auxiliary material in the prescription amount in the weighing room, and set aside.
[0041] Take 60% of the prescribed amount of fresh water for injection, let it cool to 30°C±5°C, and add the prescribed amount of histidine.
[0042] After adding the esomeprazole sodium of recipe quantity in above-mentioned solution and dissolving completely, set aside.
[0043] While stirring, add 0.5 mol / L sodium hydroxide solution to adjust the pH value of the medicinal solution within the range of 11.0-12.0.
[0044] Dilute the mixed solution to the mark with the remaining prescribed amount of water for injection (cooled to 30°C ± 5°C).
[0045] Add the specified concentration of medicinal activated carbon (unit: W / V, 0.03%), stir and absorb for 15 minutes, decarbonize and filter with 0.45um filter membrane, sterilize and filter with 0.22um filter element, measure the intermediate solution, and fill;
[0046] Freeze-drying p...
Embodiment 2
[0052]
[0053] Specific steps:
[0054] Weigh each raw and auxiliary material in the prescription amount in the weighing room, and set aside.
[0055] Take 60% of the prescribed amount of fresh water for injection, let it cool to 30°C±5°C, and add the prescribed amount of lysine.
[0056] After adding the esomeprazole sodium of recipe quantity in above-mentioned solution and dissolving completely, set aside.
[0057] While stirring, add 0.5 mol / L sodium hydroxide solution to adjust the pH value of the medicinal solution within the range of 11.0-12.0.
[0058] Dilute the mixed solution to the mark with the remaining prescribed amount of water for injection (cooled to 30°C ± 5°C).
[0059] Add the specified concentration of medicinal activated carbon (unit: W / V, 0.03%), stir and absorb for 15 minutes, decarbonize and filter with 0.45um filter membrane, sterilize and filter with 0.22um filter element, measure the intermediate solution, and fill;
[0060] Freeze-drying proc...
Embodiment 3
[0066]
[0067] Specific steps:
[0068] Weigh each raw and auxiliary material in the prescription amount in the weighing room, and set aside.
[0069] Take 60% of the prescribed amount of fresh water for injection, let it cool to 30°C±5°C, and add the prescribed amount of arginine.
[0070] After adding the esomeprazole sodium of recipe quantity in above-mentioned solution and dissolving completely, set aside.
[0071] While stirring, add 0.5 mol / L sodium hydroxide solution to adjust the pH value of the medicinal solution within the range of 11.0-12.0.
[0072] Dilute the mixed solution to the mark with the remaining prescribed amount of water for injection (cooled to 30°C ± 5°C).
[0073] Add the specified concentration of medicinal activated carbon (unit: W / V, 0.03%), stir and absorb for 15 minutes, decarbonize and filter with 0.45um filter membrane, sterilize and filter with 0.22um filter element, measure the intermediate solution, and fill;
[0074] Freeze-drying pr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com