Synthetic method of ethylene diamine derivative
A synthesis method and derivative technology, applied in the field of synthesis of ethylenediamine derivatives, can solve problems such as harsh conditions, low yield, and easy volatility, and achieve the effects of low synthesis cost, good catalytic effect, and cheap price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
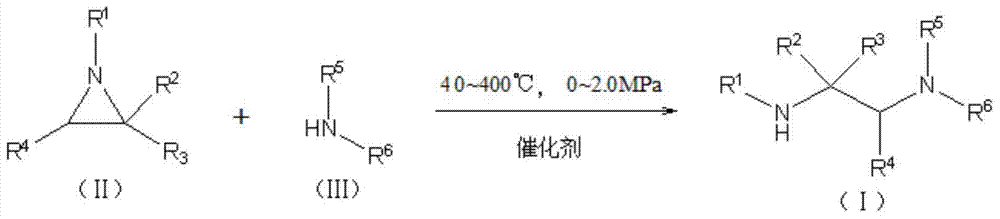
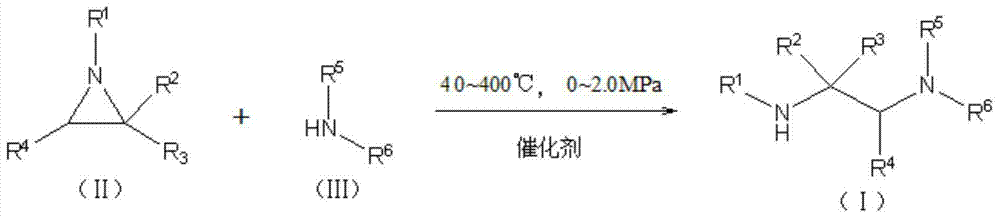
[0030] A kind of synthetic method of ethylenediamine derivative, comprises the following steps:
[0031] Use water as a solvent, add 50mmol of methylamine to a 100ml three-necked flask, add 3.20g of NKC-9 sulfonic acid cation exchange resin, stir in an oil bath at 40°C, use a low-temperature cooling liquid circulation pump for cyclic condensation, and use a constant pressure drop Aziridine 50mmol was added dropwise into the liquid funnel, and the time used for the dropwise addition process was 30min. After the dropwise addition, the reaction was stirred for 4h. After the reaction, filter, centrifuge, remove the resin, and analyze with gas chromatography, the target product yield is 98.21%.
Embodiment 2
[0033] A kind of synthetic method of ethylenediamine derivative, comprises the following steps:
[0034] Use water as a solvent, add 50mmol of dimethylamine to a 100ml three-necked flask, add 3.20g of NKC-9 sulfonic acid cation exchange resin, stir in an oil bath at 40°C to 44°C, and use a low-temperature cooling liquid circulation pump to perform cyclic condensation. Add 50 mmol of 2-ethylaziridine dropwise with a constant-pressure dropping funnel. The time for the dropwise addition is 30 minutes. After the dropwise addition, the temperature of the oil bath is raised to 52° C., and the reaction is stirred for 6 hours. After the reaction was finished, filter, centrifuge, remove the resin, and analyze by gas chromatography, the target product yield was 96.23%. Example 3
Embodiment 3
[0035] A kind of synthetic method of ethylenediamine derivative, comprises the following steps:
[0036] Using water as a solvent, add 150mmol of dimethylamine to a 100ml three-necked flask, add 1.60g of NKC-9 sulfonic acid cation exchange resin, stir in an oil bath at 40°C to 44°C, and use a low-temperature cooling liquid circulation pump to carry out cyclic condensation. Add 50 mmol of 1-phenylaziridine dropwise with a constant-pressure dropping funnel, and the time for the dropwise addition is 30 minutes. After the dropwise addition, the temperature of the oil bath is raised to 52° C., and the reaction is stirred for 6 hours. After the reaction, filter, centrifuge, remove the resin, and analyze with gas chromatography, the target product yield is 80.72%. Example 4
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com