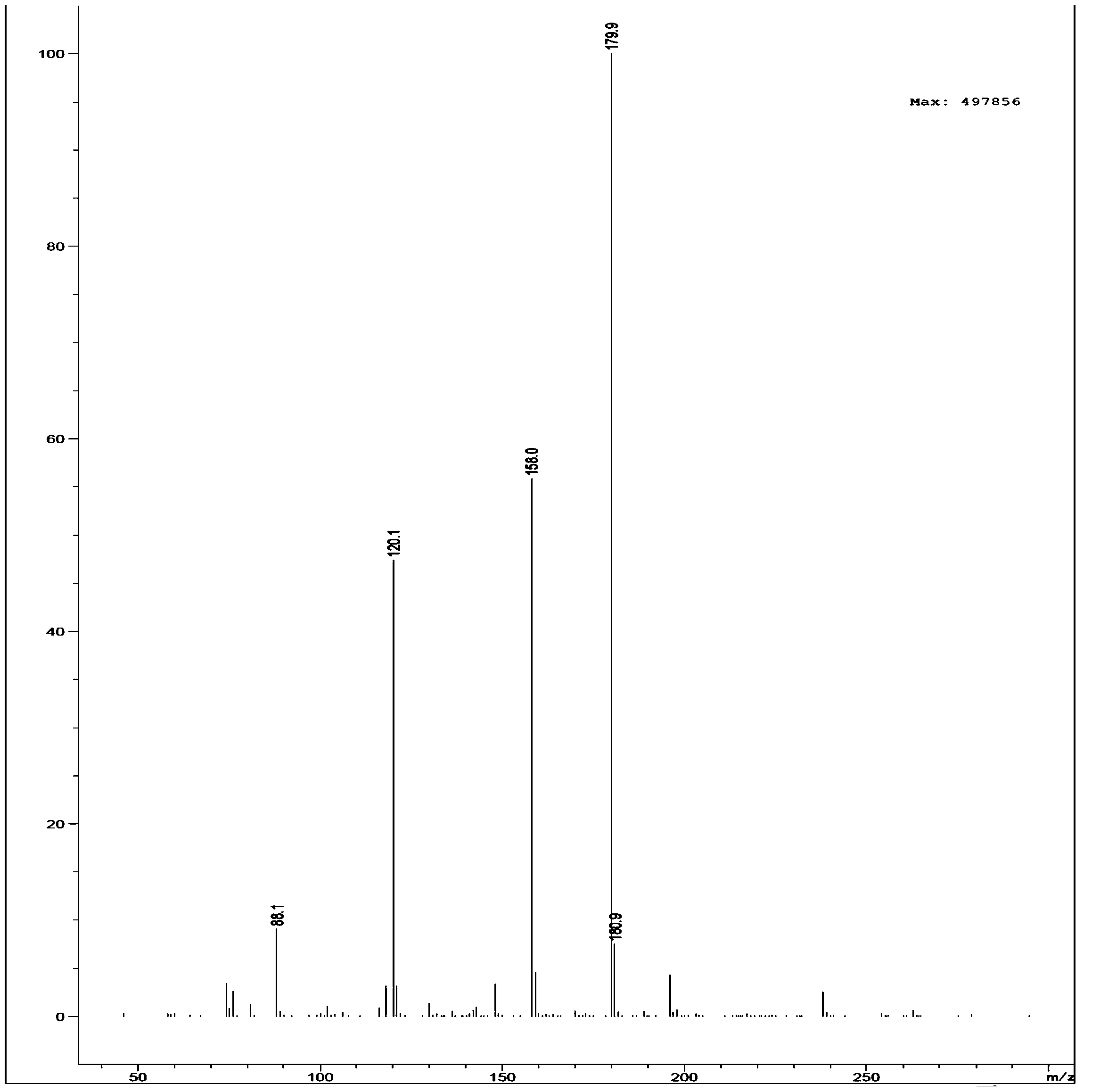
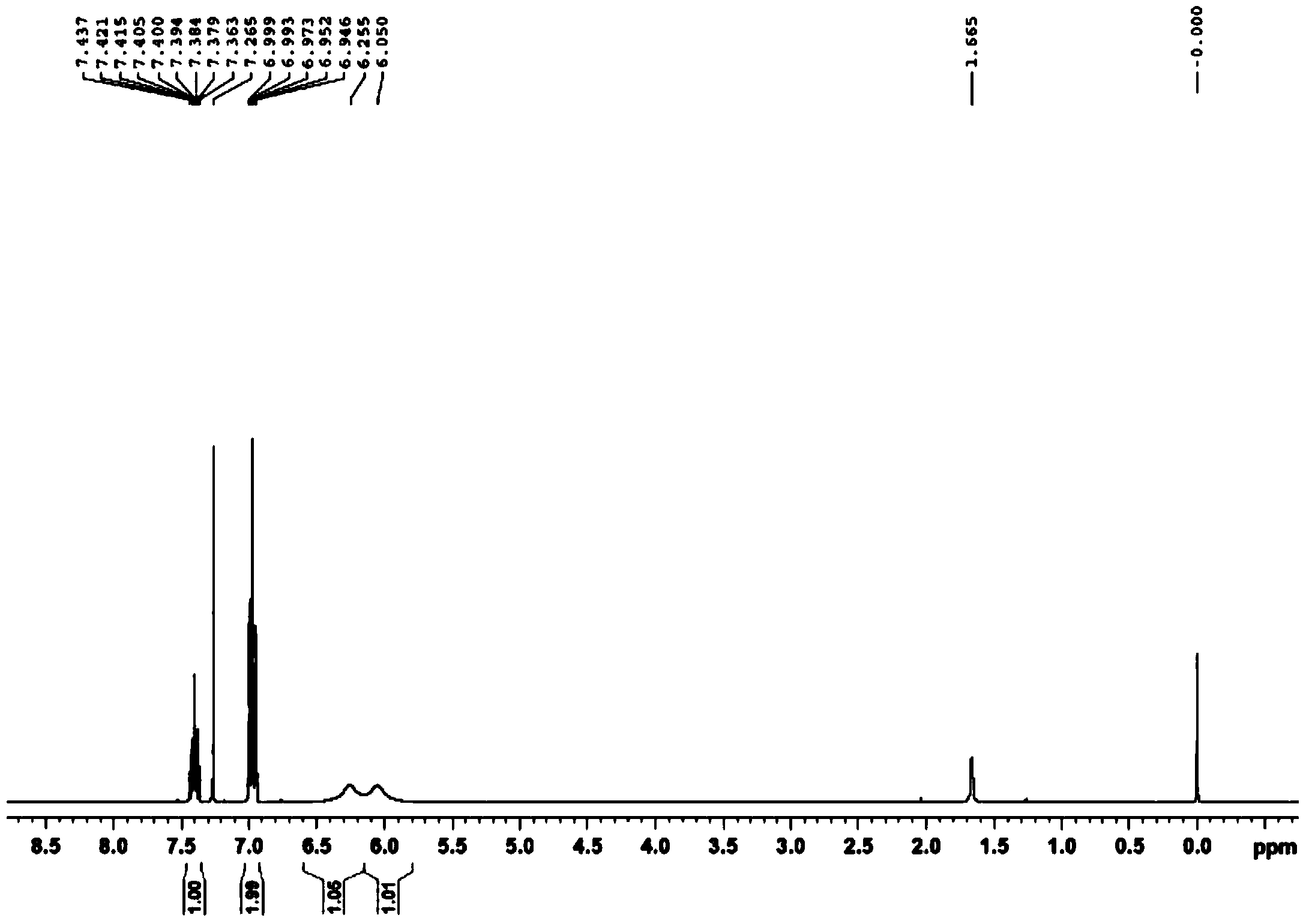
Preparation method of 2,6-difluorobenzamide by utilizing rhodococcus ruber
A technology of difluorobenzamide and Rhodococcus erythrococcus, applied in the biological field, can solve problems such as high requirements for production equipment and production conditions, low conversion rate and selectivity of the method, and harsh reaction conditions of the production process, so as to reduce production costs, The effect of high substrate tolerance and fast reaction speed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] A kind of method utilizing Rhodococcus rubrum to prepare 2,6-difluorobenzamide, the preparation steps are:
[0030] (1) Seed and fermentation culture: inoculate the strain of Rhodococcus ruber (CGMCC3090) into the seed medium, culture at 180r / min, 30°C for 28h to obtain the seed culture medium, and transfer it to fresh fermentation medium with an inoculation amount of 3wt%. Culture medium, 180r / min, 28°C, cultured for 48h, to obtain bacterial fermentation liquid:
[0031] Seed medium: glycerol 10g / L, peptone 5g / L, malt powder 3g / L, yeast powder 3g / L, NaCl 1g / L, pH7.0;
[0032] Fermentation medium: glucose 20g / L, yeast powder 5g / L, MgSO 4 ·7H 2 O. KH 2 PO 4 、K 2 HPO 4 0.5g / L, urea 7g / L, NaCl 2g / L, CoCl 2 0.02g / L, sodium glutamate 1g / L, pH7.0. Among them, urea and inducer CoCl2 are sterilized separately for 3-5 minutes.
[0033] (2) Preparation of Rhodococcus Erythrococcus Suspension: Centrifuge the above bacterial fermentation liquid, remove the supernatant, and...
Embodiment 2
[0040] In the present embodiment, steps (1), (2) and (4) are all the same as in Example 1, and only step (3) is described below.
[0041] (3) Conversion of 2,6-difluorobenzonitrile: the concentration of substrate 2,6-difluorobenzonitrile is 1mol / L, the reaction volume is 10ml, the final concentration of Rhodococcus cells is 2.37g / L, and the final concentration of adding is 1% methanol, conversion system is 50mmol / LpH7.5KH 2 PO 4 -NaOH buffer solution, 30°C, 180r / min, after conversion for 3 hours, the pure product of 2,6-difluorobenzamide was prepared.
[0042] It is detected that the conversion rate of 2,6-difluorobenzonitrile in this step reaches 100%, and the selectivity to 2,6-difluorobenzamide is 100%.
Embodiment 3
[0044] In the present embodiment, steps (1), (2) and (4) are all the same as in Example 1, and only step (3) is described below.
[0045] (3) Conversion of 2,6-difluorobenzonitrile: the concentration of substrate 2,6-difluorobenzonitrile is 2.5mol / L, the reaction volume is 10ml, the final concentration of Rhodococcus cells is 2.37g / L, and 1% of Tween 80, the conversion system is 50mmol / LpH7.5KH 2 PO 4 -NaOH buffer solution, 30°C, 180r / min, after conversion for 24h, pure 2,6-difluorobenzamide was prepared.
[0046] It is detected that the conversion rate of 2,6-difluorobenzonitrile in this step reaches 100%, and the selectivity to 2,6-difluorobenzamide is 100%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com