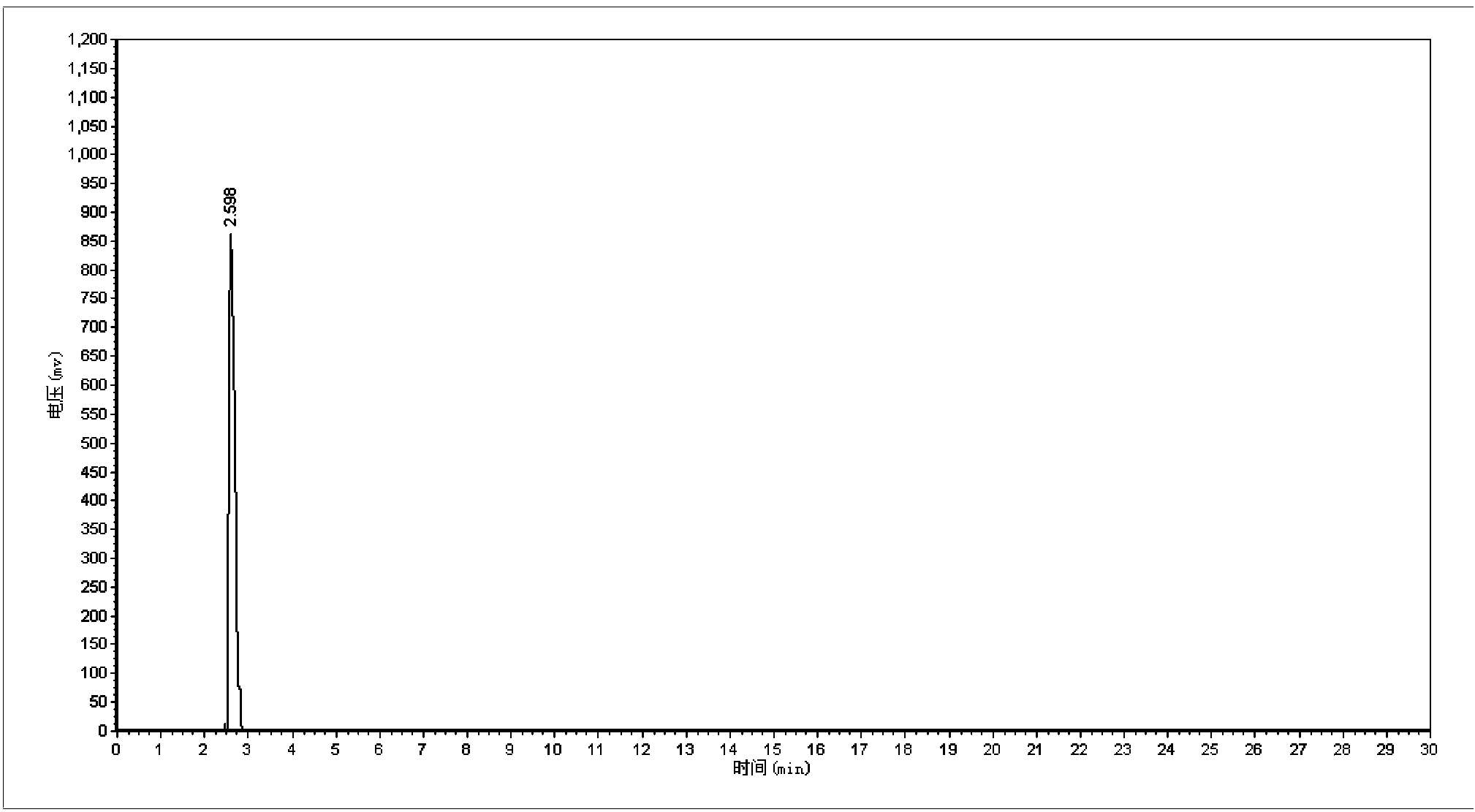
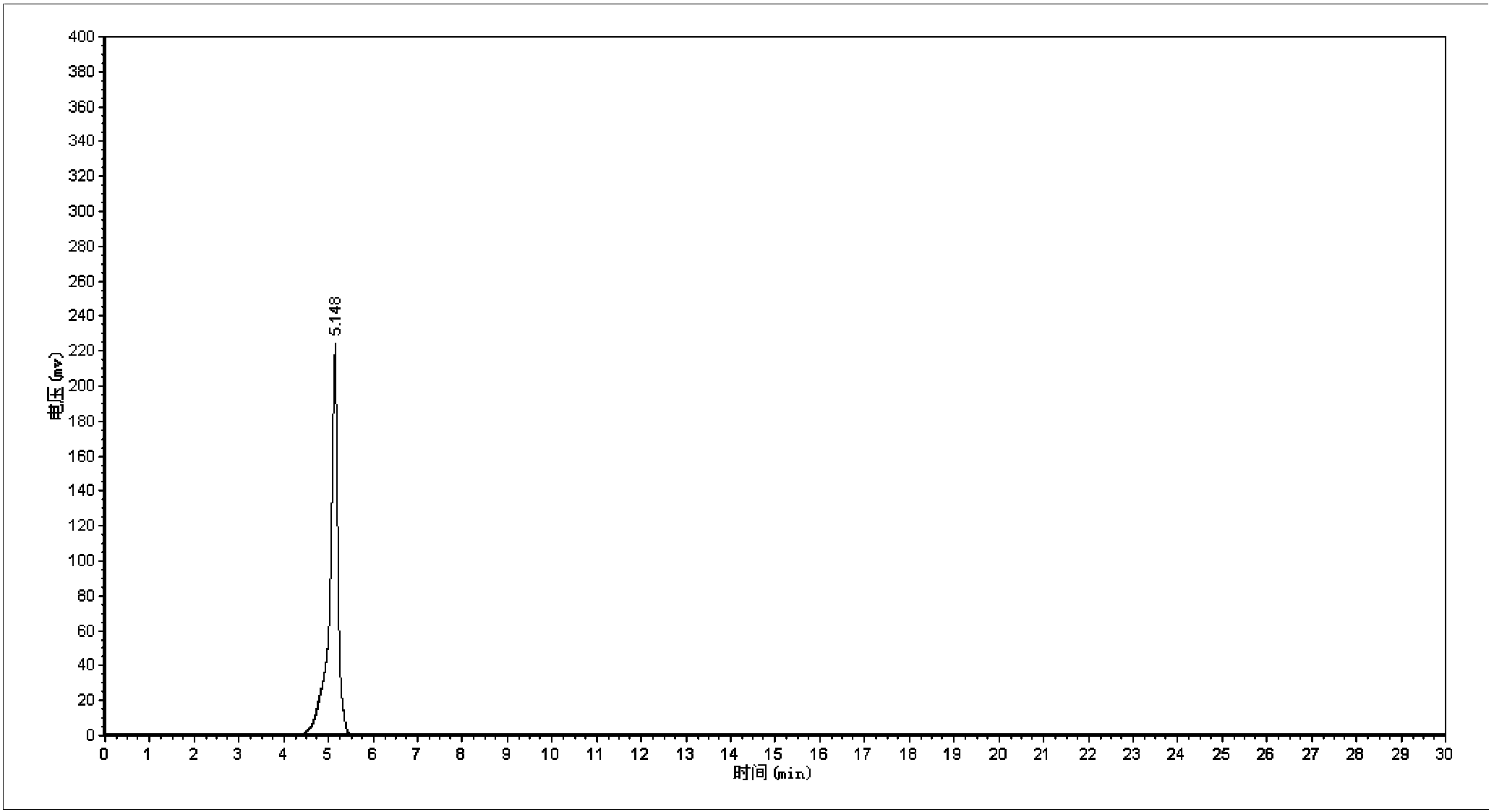
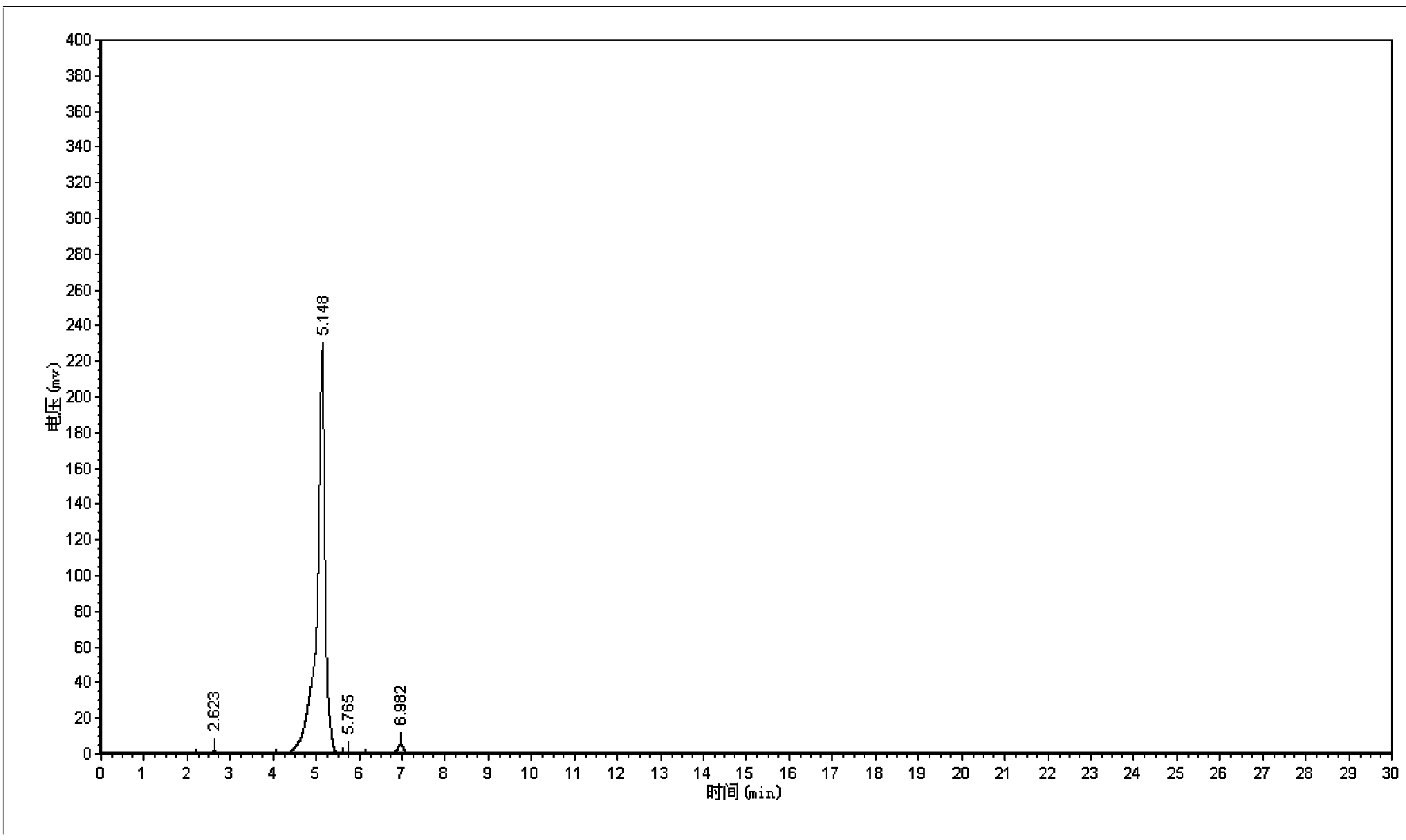
Synthetic method of 5-cholro-8-quinoline oxyacetic acid
A technology of quinolineoxyacetic acid and a synthesis method, which is applied in the field of synthesis of 5-chloro-8-quinolineoxyacetic acid, can solve the problems of environmental pollution in the chlorination step, corroded equipment, low yield and the like, and avoids safety Problems and environmental pollution problems, mild production process conditions, high product yield effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
[0020] Specific embodiment 1: A method for synthesizing 5-chloro-8-quinolinoxyacetic acid in this embodiment is completed by the following steps:
[0021] 1. Mix 8-quinolinoxyacetic acid with an aqueous hydrochloric acid solution with a mass percentage of 12%-28% to prepare an 8-quinolinoxyacetic acid electrolyte with a concentration of 5~150g / L; 2. 1. The prepared electrolyte is placed in a single-chamber electrolytic cell, with platinum plates as anode and cathode electrodes, and electrolytic chlorination is carried out for 0.5 to 5 hours under the condition of current intensity of 0.2 to 0.8A; The hydrochloric acid is removed by pressure distillation to obtain crude 5-chloro-8-quinolinoxyacetic acid, and then the crude 5-chloro-8-quinolinoxyacetic acid is refined by recrystallization method to obtain 5-chloro-8-quinoline Pure oxyacetic acid.
specific Embodiment approach 2
[0022] Specific embodiment two: this embodiment is different from specific embodiment one in that the concentration of 8-quinolinoxyacetic acid in step two is 20-120 g / L. Others are the same as the first embodiment.
specific Embodiment approach 3
[0023] Specific embodiment three: This embodiment is different from specific embodiment one or two in that the concentration of 8-quinolinoxyacetic acid in step two is 50-120 g / L. Others are the same as the first or second embodiment.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com