Synthetic method of 3-(beta-hydroxyethyl sulfonyl)-nitrobenzene
A hydroxyethylsulfone-based, synthetic method technology, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry and other directions, can solve the problems of increasing the difficulty of post-processing, reducing the quality of recovered products, and long reaction time, avoiding high temperature The effect of concentration operation, reduction of raw material input cost, and simplification of post-processing operations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
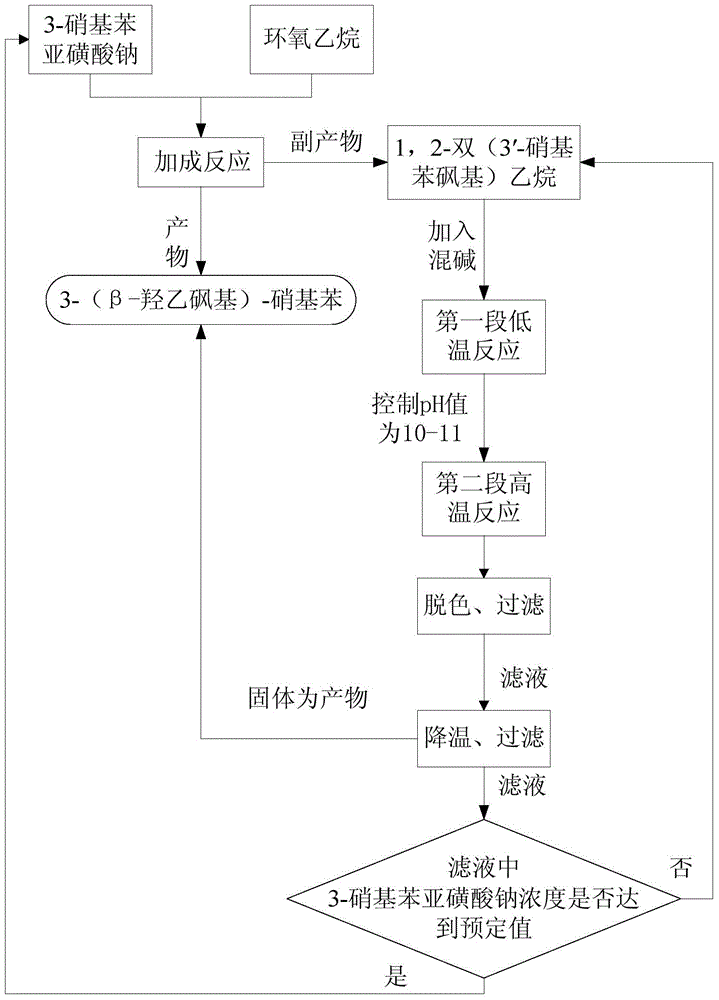
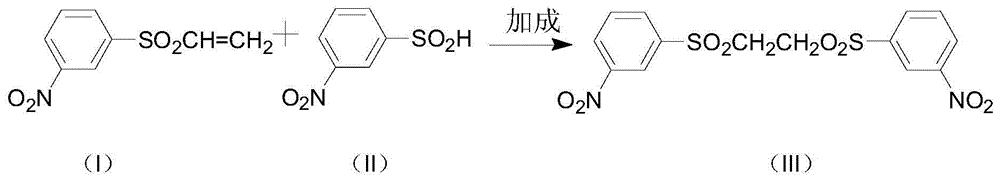
[0025]In a 2000mL flask, add 360g of sodium 3-nitrobenzenesulfinate, 1000mL of water, dropwise add 160g of ethylene oxide, neutralize with dilute sulfuric acid throughout the dropping process, control the pH range of 7.0-7.5, at 60°C Reacted for 10 hours. After the reaction was completed, it was vacuum filtered through a Buchner funnel and washed with water to obtain 395 g of filter cake 3-(β-hydroxyethylsulfone)-nitrobenzene, which was extracted with methanol to obtain "bissulfone" (2-bis( 3'-nitrophenylsulfone) ethane) 38g, prepare 1000g of 2-bis(3'-nitrophenylsulfone)ethane according to the same method, for the following hydrolysis reaction.
Embodiment 2
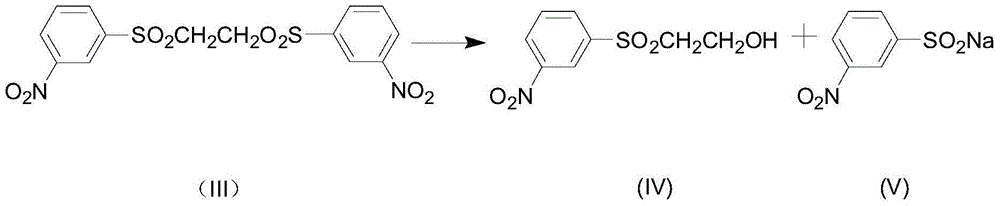
[0027] In a 1000mL flask, add 400g of clear water, 13g of liquid caustic soda with a concentration of 30% by mass, 1.5g of sodium carbonate, 44.4g of 2-bis(3′-nitrophenylsulfone)ethane, and the pH value of the system is greater than 14, stir and heat At 70°C, heat-retain for 1 hour. At this time, the bissulfone has been partially hydrolyzed. The pH value of the reaction system measured at the end of the heat-preservation is 9.6. Add 2 g of sodium carbonate to adjust the pH value to 11. Continue to heat up to 98°C. After 3 hours of heat preservation The bissulfone is completely hydrolyzed, there is no oil layer at the bottom of the flask, the reaction liquid is bright yellow, and there is basically no white insoluble matter. Cool down to 80°C, add 0.3g activated carbon for decolorization, stir for 0.5h, filter, stir the filtrate and cool down to 20°C, filter, solid Dry to obtain 21.5g of 3-(β-hydroxyethylsulfonyl)-nitrobenzene, the yield is 83.8%, the purity is 98.7%, the filtra...
Embodiment 3
[0029] In a 1000mL three-necked flask, add 400mL of the mother liquor of Example 2, add 12g of liquid caustic soda and 2g of soda ash with a mass percentage concentration of 30%, and 44.4g of 2-bis(3'-nitrophenylsulfone)ethane, the first Section heat preservation reaction temperature is 75 DEG C, other adopts the same method as embodiment 2 to operate, the pH value is 10.9 after the first hour heat preservation finishes, continue to heat up to 100 DEG C, heat preservation 3h, phenomenon is consistent with embodiment 1, there is no Black oil layer. After a series of operations such as cooling, decolorization and filtration, 20.8 g of dry product 3-(β-hydroxyethylsulfone group)-nitrobenzene was obtained, the purity was 98.3%, and the yield was 81.1%; the filtrate was 422 g, and the mass content of sodium sulfinate was measured as 9.5%, namely 40.1g, the filtrate continues to apply mechanically to the next batch of hydrolysis. After applying mechanically to the third batch, the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com