A nitrogen-doped carbon-oxygen reduction catalyst containing iron ferric oxide particles and its preparation method
A technology of ferroferric oxide and nitrogen-doped carbon, which is applied in the field of catalyst research, can solve the problems of poor oxygen reduction reaction activity, difficulty in recombining ferric oxide and nitrogen-doped carbon, etc., to improve the catalytic activity of oxygen reduction reaction, The preparation process is simple and easy, and the effect of better stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
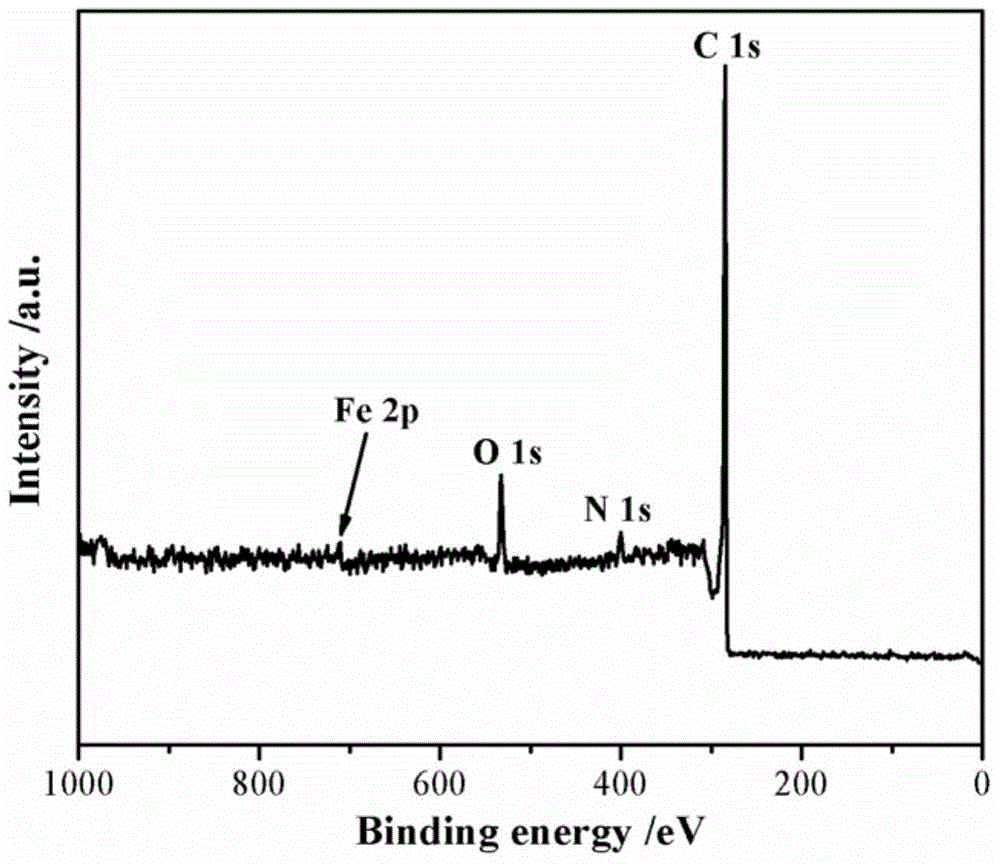
Image
Examples
Embodiment 1
[0025] Dissolve 201.3mg of pyrrole monomer and 801.9mg of ferric chloride hexahydrate in 30mL of absolute ethanol respectively, and mix them ultrasonically for 40min. The obtained mixed solution was transferred into a 100mL autoclave, and reacted solvothermally at 180°C for 12h. Then it was naturally cooled to room temperature, the solvothermal product was washed with ethanol centrifugation for 3 times, and dried at 60°C for 12h. The dried solid sample was pyrolyzed at 1000°C in an argon atmosphere and kept for 4 hours. Then cool naturally to room temperature to obtain nitrogen-doped carbon catalyst I containing ferric oxide particles.
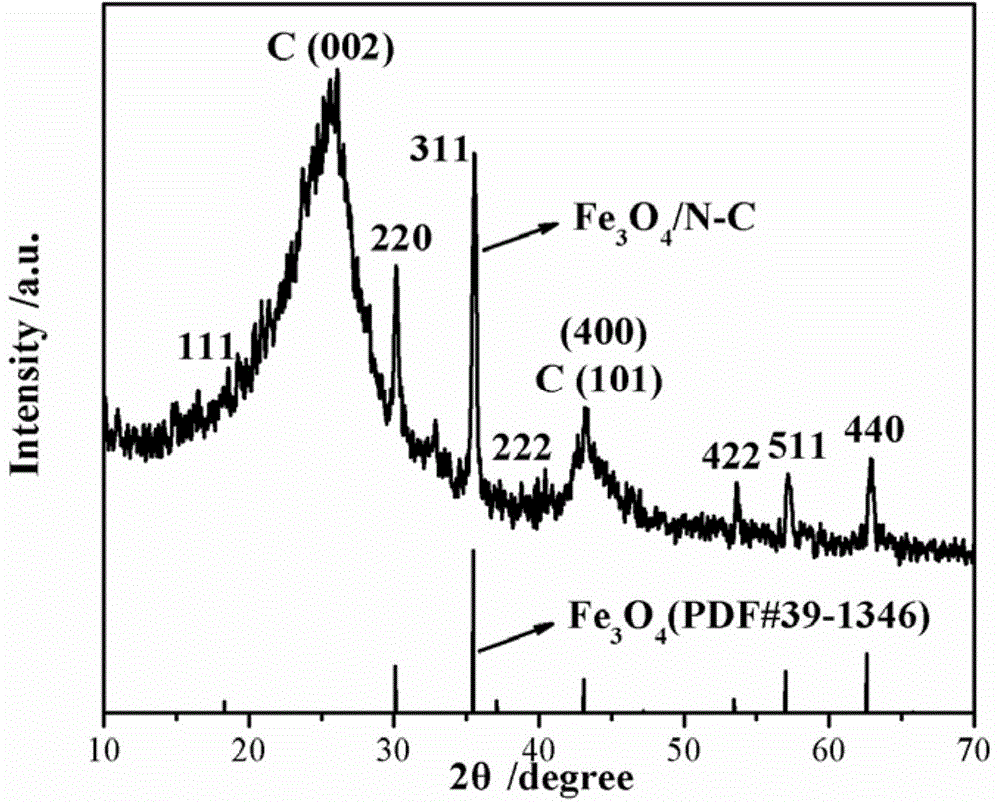
[0026] Its X-ray diffraction result of catalyst I prepared in embodiment 1 can be found in figure 1 . Depend on figure 1 It can be seen that the catalyst I prepared in Example 1 contains carbon and ferric oxide in the components.
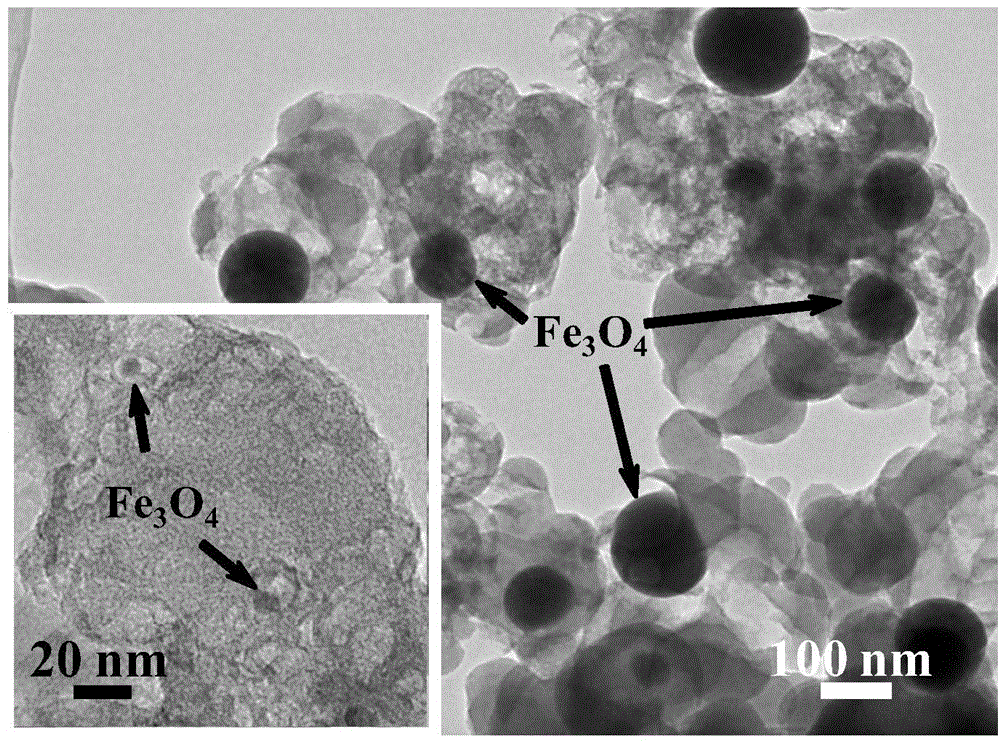
[0027] The scanning electron microscope result of the catalyst I prepared in embodiment 1 can be found in ...
Embodiment 2
[0033] Replace "201.3 mg of pyrrole monomer and 801.9 mg of ferric chloride hexahydrate in 30 mL of absolute ethanol" with "400 mg of pyrrole monomer and 400 mg of ferric chloride hexahydrate respectively in 30 mL of absolute ethanol" Dissolved in 30mL absolute ethanol", replace the "dried solid sample at 1000°C in an argon atmosphere for high temperature pyrolysis" in Example 1 with "the dried solid sample is subjected to high temperature pyrolysis in an argon atmosphere Carrying out high temperature pyrolysis", the other steps and conditions were the same as in Example 1, and nitrogen-doped carbon catalyst II containing ferric oxide particles was prepared.
[0034] The test method for the oxygen reduction activity of the nitrogen-doped carbon catalyst II containing ferric oxide particles prepared in Example 2 is the same as that in Example 1. The results show that the linear sweep voltammetry test result of the catalyst II prepared in embodiment 2 can be found in Figure 4 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com