Ropinirole-containing adhesive skin patch and packaged product thereof
一种贴附剂、游离体的技术,应用在含有罗匹尼罗的贴附剂及其包装体领域,能够解决罗匹尼罗的皮肤透过性不充分、症状日间变化强烈等问题,达到皮肤透过性优异、充分粘着性、经时稳定性优异的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0101] First, using a mixer, 10.0 parts by mass of ropinirole hydrochloride (8.8 parts by mass converted into ropinirole free body), 1.1 parts by mass of sodium hydroxide (desalting agent), 29.6 parts by mass of liquid paraffin, toluene (solvent ), 29.6 parts by mass of styrene-isoprene-styrene block copolymer (SIS) (SIS5000, manufactured by JSR Corporation), and 29.6 parts by mass of alicyclic hydrocarbon resin were mixed to obtain an adhesive layer composition. The obtained pressure-sensitive adhesive layer composition was spread on a release sheet formed of a film (made of polyester) subjected to mold release treatment with silicone, and the toluene was removed by drying to form a pressure-sensitive adhesive layer. Next, a film (made of polyester) was laminated as a support layer on the surface of the pressure-sensitive adhesive layer opposite to the release sheet to obtain an adhesive patch. Table 1 shows the composition (excluding toluene) of the above pressure-sensitive ...
Embodiment 2
[0102] (Example 2, Comparative Examples 1-2)
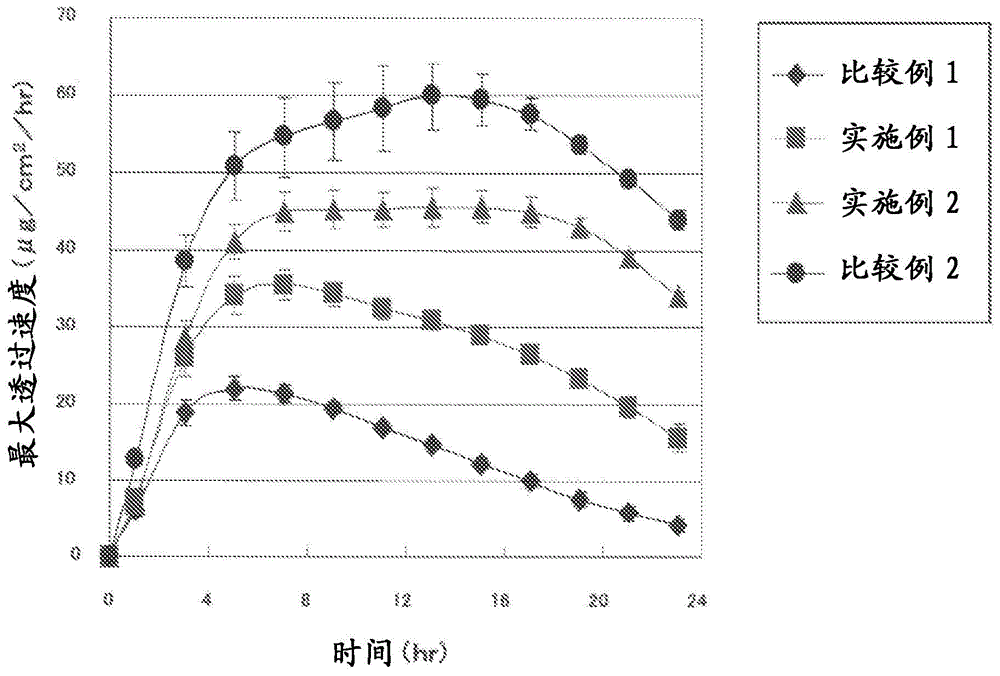
[0103] Except having made the composition of the adhesive layer composition into the composition shown in Table 1, it carried out similarly to Example 1, and obtained the patch. With regard to the patch preparations obtained in Examples 1-2 and Comparative Examples 1-2, a skin penetration test and an evaluation test of cohesiveness of the adhesive layer were performed, respectively. The results of the cohesion evaluation test of the adhesive layer are shown in Table 1 together with the composition of the adhesive layer composition in each example and comparative example, and the results of the skin penetration test are shown in Table 1. figure 2 middle.
[0104] [Table 1]
[0105]
[0106] From Table 1 and figure 2 The results shown clearly confirm that the patch of the present invention has excellent skin permeability of ropinirole, and that the cohesive force of the adhesive layer is strong, so that sufficient adhesivene...
Embodiment 3~5
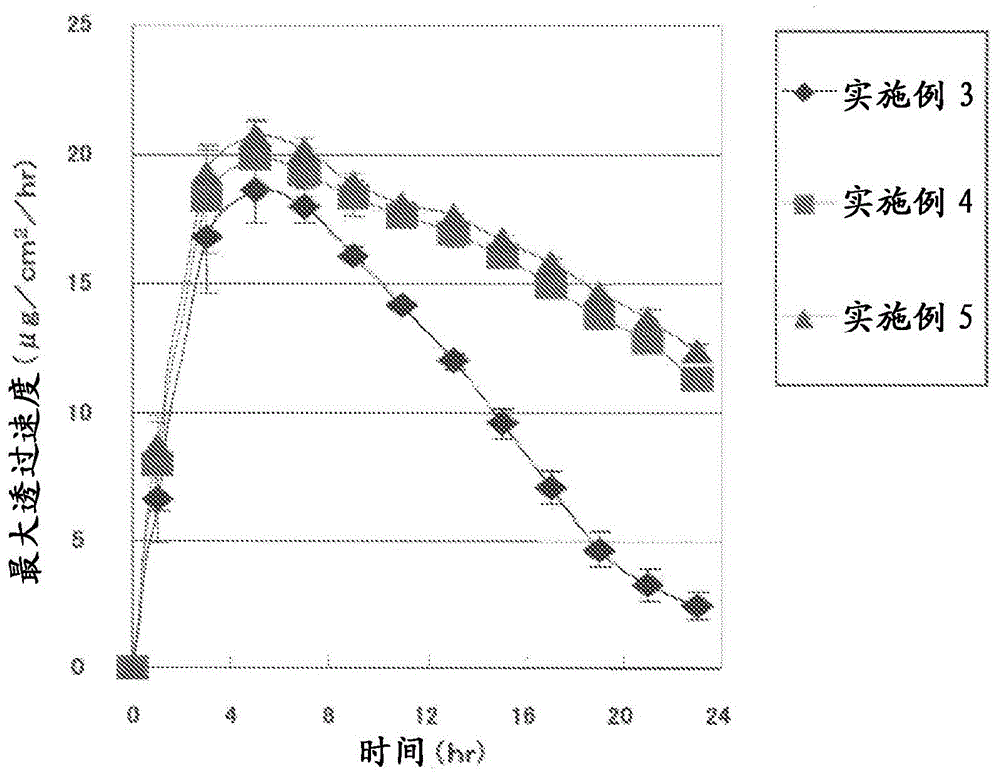
[0108] The composition of the adhesive layer composition was the composition shown in Table 2, and the mass per unit area of the adhesive layer was the mass shown in Table 2, respectively, and the stickers were obtained in the same manner as in Example 1. Attachment. With regard to the patches obtained in Examples 3 to 5, a skin permeation test and a cohesiveness evaluation test of the adhesive layer were respectively performed. The results of the cohesion evaluation test of the adhesive layer are shown in Table 2 together with the composition of the adhesive layer composition in each example, and the results of the skin penetration test are shown in Table 2. image 3 middle.
[0109] [Table 2]
[0110]
[0111] From Table 2 and image 3 The results shown clearly confirm that the patch of the present invention has excellent skin permeability of ropinirole, and that the cohesive force of the adhesive layer is strong, so that sufficient adhesiveness can be exhibited. In...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com