An external skin patch containing flupirtine or a pharmaceutically acceptable salt thereof
A technology of medicinal salt and flupirtine, applied in the field of pharmaceutical preparations, can solve the problems of poor bioavailability, low compliance, high blood drug concentration, etc., to improve skin permeability, improve stability, and ensure high effect of concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
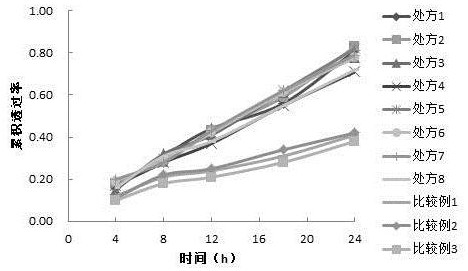
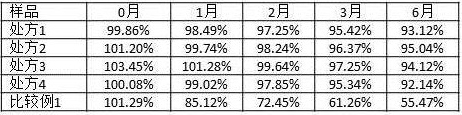
Image
Examples
Embodiment 1
[0089] Example 1 Self-adhesive matrix layer patch containing acrylate pressure-sensitive adhesive
[0090] Table 1. Formulation of Self-Adhesive Matrix Patches Containing Acrylate Pressure Sensitive Adhesives
[0091]
[0092] Recipe 1: Take the acrylate pressure-sensitive adhesive in the prescribed amount, add 6 parts of flupirtine maleate and 15 parts of solubilizer PVP, mix well, add 18 parts of penetration enhancer azone and 3 parts of antioxidant and sodium metabisulfite, Add an appropriate amount of methanol, mix evenly, add 1 part of sodium bicarbonate, stir evenly to obtain a paste, coat it on the support layer, dry, and after the organic solvent is volatilized, cover the release liner to obtain a transdermal absorption preparation.
[0093] Formulation 2: The difference from Formulation 1 is that each substance was added according to Table 1, and a transdermal absorption preparation was prepared in the same manner as Formulation 1.
[0094] Formulation 3: The diff...
Embodiment 2
[0105] Example 2 Self-adhesive matrix layer patch containing polyisobutylene pressure-sensitive adhesive
[0106] Table 4. Formulation of self-adhesive matrix layer patch containing polyisobutylene pressure-sensitive adhesive
[0107]
[0108] Recipe 5: Take the polyisobutylene pressure-sensitive adhesive in the prescribed amount, add 10 parts of light liquid paraffin, 3 parts of colloidal silicon dioxide, after mixing evenly, add 5 parts of PVP, 5 parts of menthol, 5 parts of azone and an appropriate amount of methanol , stir and mix evenly, add 1 part of flupirtine maleate to the above mixing system, after the drug is completely dissolved, add 0.3 part of sodium bicarbonate, stir evenly, and then get a paste, spread it on the support layer, dry , and after the organic solvent is volatilized, the release liner is covered to obtain a transdermal absorption preparation.
[0109] Formulation 6: The difference from Formulation 4 is that each substance is added according to Ta...
Embodiment 3
[0122] Example 3 A patch containing a self-adhesive matrix layer of silicone pressure-sensitive adhesive
[0123] Table 7. Patch Formulations for Self-Adhesive Matrix Layers Containing Silicone Pressure Sensitive Adhesives
[0124]
[0125]Recipe 9: Take the silicone pressure-sensitive adhesive in the prescribed amount, add 10 parts of light liquid paraffin, 3 parts of colloidal silicon dioxide, mix well, add 15 parts of PVP, 5 parts of menthol, 5 parts of azone and an appropriate amount of methanol , stir and mix evenly, add 6 parts of flupirtine maleate to the above mixing system, after the drug is completely dissolved, add 0.5 part of sodium bicarbonate, stir evenly, and then get a paste, coat it on the support layer, dry , and after the organic solvent is volatilized, the release liner is covered to obtain a transdermal absorption preparation.
[0126] Formulation 10: The difference from Formulation 9 is that each substance is added according to Table 7, the solubilize...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com