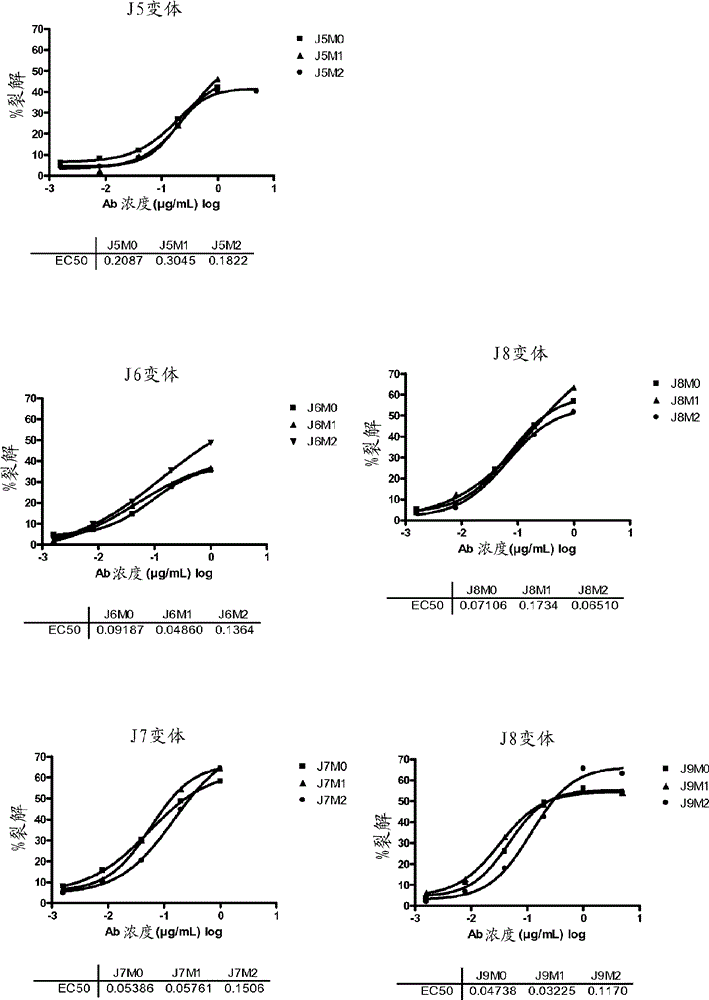
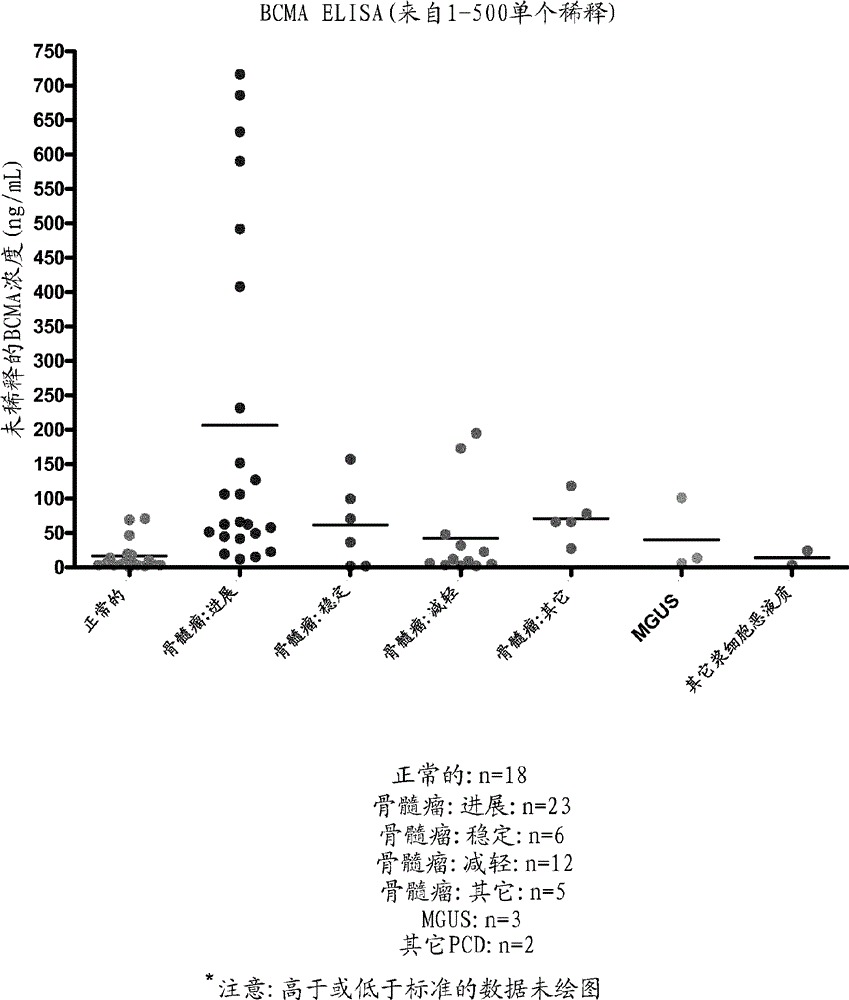
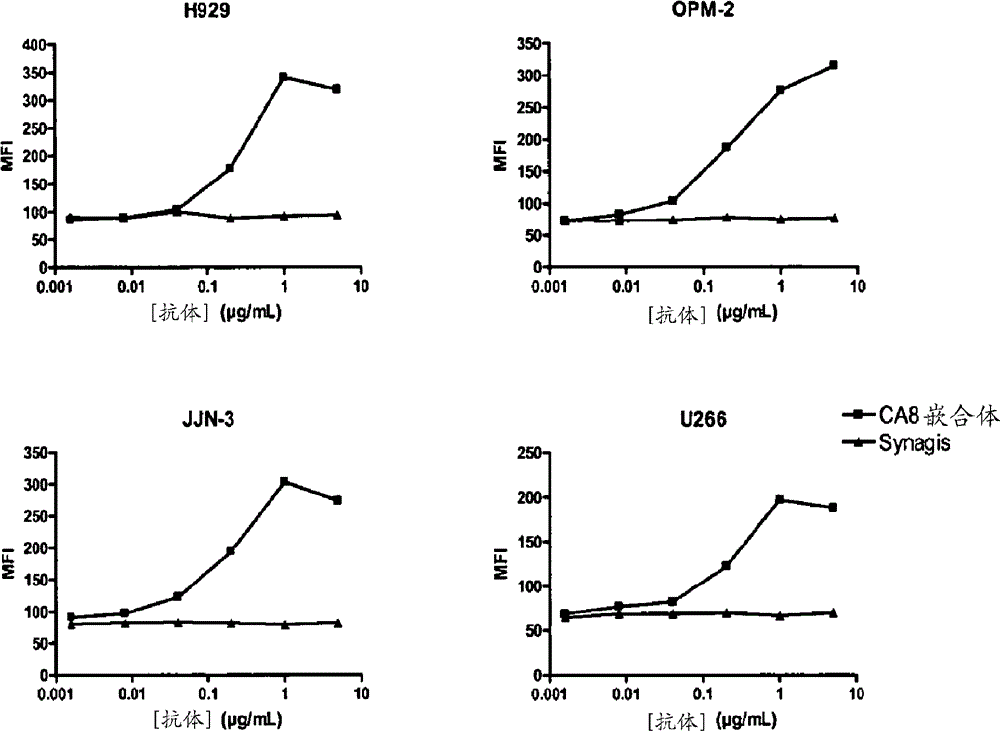
BCMA (CD269/TNFRSF17) binding proteins
A protein-binding and fragment-binding technology, applied in the field of human BCMA (hBCMA) antigen-binding protein and its fragments, can solve problems such as low expression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0277] Preparation of ADCs:
[0278] In antibody drug conjugates, the antibody can be conjugated to a cytotoxic agent either directly or via a linker. Suitable linkers include, for example, cleavable and non-cleavable linkers. A cleavable linker is typically readily cleavable under intracellular conditions. Suitable cleavable linkers include, for example, peptide linkers cleavable by intracellular proteases, such as lysosomal or endosomal proteases. In an exemplary embodiment, the linker may be a dipeptide linker, such as a valine-citrulline (val-cit) or phenylalanine-lysine (phe-lys) linker. Other suitable linkers include linkers that are hydrolyzable at pH below 5.5, such as hydrazone linkers. Additional suitable cleavable linkers include disulfide linkers.
[0279] Bristol-Myers Squibb have described specific lysosomal enzyme-cleavable antineoplastic drug conjugates. See, eg, US Patent No. 6,214,345. Seattle Genetics has published applications US Patent Application No...
Embodiment 1
[0302] Example 1 Production and Selection of Monoclonal Antibodies
[0303] 1.1 Immunization strategy
[0304] The anti-human BCMA mAb murine parental CA8 was identified from a hybridoma derived from mice immunized with full-length human BCMA. BALB / c mice were immunized intraperitoneally with 25 μg of recombinant (rBCMA) protein in combination with CFA. Mice were boosted three times at one-month intervals with 25 μg full-length rBCMA protein + 10 μg monophosphoryl lipid A-stabilized emulsion (MPL-SE) (Corixa Corporation, Seattle, WA) and administered intravenously 3 days before fusion 30 μgrBCMA protein was boosted before the fusion. Hybridomas can be generated and cloned using the ClonaCell-HY Hybridoma Cloning Kit (StemCell Technologies, Vancouver, BC) or using conventional methods. In a conventional approach, B cells from the spleen of immunized animals were fused with Sp2 / 0 myeloma cells in the presence of PEG (Sigma-Aldrich, St. Louis, MO). After overnight recovery, c...
Embodiment 2
[0311] 2.1 Cloning of variable region of CA8 hybridoma
[0312] Total RNA was extracted from CA8 hybridoma cells, followed by reverse transcription and polymerase chain reaction (RT-PCR) to generate light chain variable domain cDNA sequences. The forward primer used for RT-PCR was a mixture of degenerate primers specific for the leader sequence of the murine immunoglobulin genes, and the reverse primer was specific for the antibody constant region. Reverse primers specific for IgGl, IgG2a and IgG2b were used in this example since the isotypes were unknown. To design primers, mouse V H and V k DNA Multiple Sequence Alignment of Gene Leader Sequences.
[0313] 2.2 Cloning of chimeric CA8
[0314] DNA expression constructs encoding chimeric antibodies were made de novo by constructing overlapping oligonucleotides including restriction sites for cloning into mammalian expression vectors as well as human signal sequences. Will Hind III and SpeI Restriction sites were intro...
PUM
| Property | Measurement | Unit |
|---|---|---|
| affinity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com