Diagnostic markers and therapeutic targets for Kawasaki disease
A technology for Kawasaki disease and biomarkers, which can be used in disease diagnosis, biological testing, biomaterial analysis, etc., and can solve problems such as increased morbidity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0088] Example 1. Study Design, Participants and Results
[0089] The study was conducted in two periods. For the discovery period, urine samples from 6 patients with KD (including those with or without coronary artery dilatation) were compared with urine samples from 6 patients who were initially suspected of having KD but eventually diagnosed with a febrile illness mimicking KD. Compare. The discovery analysis also included 3 inter-individual control samples collected from KD patients after completion of treatment and resolution of symptoms. The validation phase recruited patients for whom evaluation for possible KD was made, but before a final diagnosis was made. Serum samples collected from an independent validation cohort collected as part of the Pediatric HeartNet Final Study of Kawasaki Disease. See Newburger et al., 356 New Engl. J. Med. 663 (2007).
[0090] Urine was collected as a clean collection sample for all study patients. Samples were labeled with study nu...
Embodiment 2
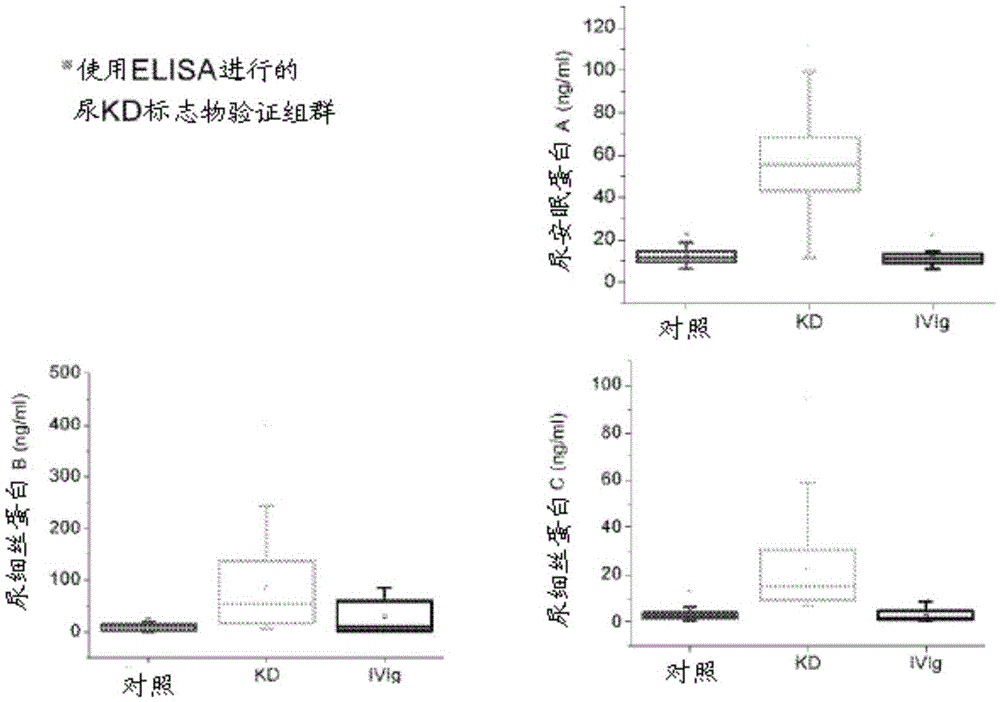
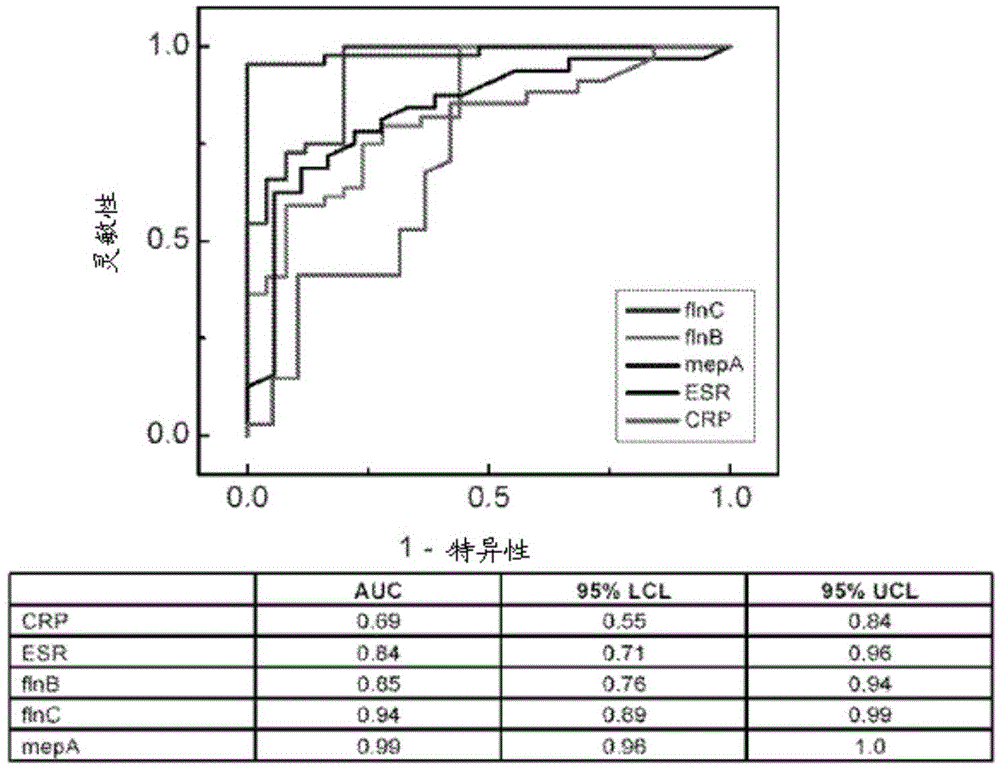
[0093] Example 2. Urine protein analysis and immunoassay
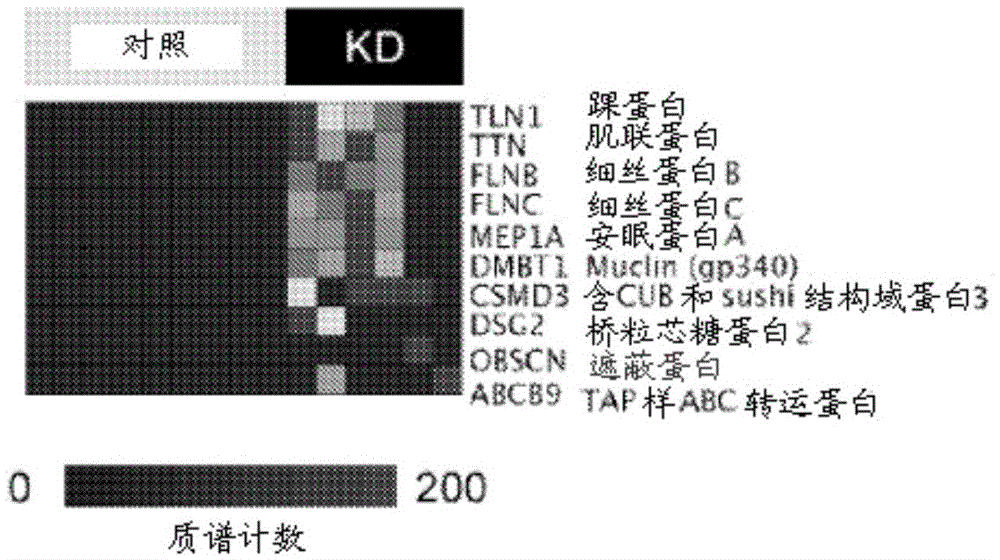
[0094] To discover candidate markers of KD, thawed 5 ml urine aliquots were fractionated using ultracentrifugation, protein precipitation, SDS-polyacrylamide gel electrophoresis, and reverse-phase liquid chromatography, as published in detail. Kentsis et al., 3 Proteomics Clin. Appl. 1052 (2009). Individual urine protein fractions were subjected to liquid chromatography tandem using a nanoflow HPLC system (Eksigent, Dublin, CA) coupled to a hybrid linear ion trap-Fourier transform ion cyclotron resonance mass spectrometer (LTQFT Ultra, Thermo Scientific, Waltham, MA). mass spectrum. For each MS / MS spectrum, the 200 strongest peaks were extracted by using MASCOT (version 2.1.04, Matrix Science) against the Human International Protein Index database (version 3.69, http: / / www.ebi.ac.uk / IPI ) to search. Assessment of identification accuracy was performed by searching the bait database consisting of the reversed protein...
Embodiment 3
[0104] Example 3. Urinary model of coronary arteries and meprin A immunohistochemistry
[0105] An established murine model of coronary arteritis based on intraperitoneal injection of group B Lactobacillus casei cell wall extract (LCWE). Lehman et al., 48 Clin. Immunol. Immunopathol. 108 (1988). L. casei group B was grown and cell wall extracts (LCWE) were prepared as described. Schulte et al., 183 J. Immunol 5311 (2009). Briefly, 6-week-old C57 / BL6 mice were injected with 250 μg LCWE in phosphate-buffered saline (PBS) or with saline alone. After 14 days, mice were sacrificed and coronary arteritis was identified in serial sections (7 [mu]m), fixed with formalin and stained with hematoxylin and eosin. For immunohistochemical analysis, sections were pretreated with 0.3% PBS in hydrogen peroxide for 30 min. Meprin A (clone F-20, Santa Cruz Biotech., Santa Cruz, CA) or isotype control antibody (goat serum, Santa Cruz Biotech.) was used 1:100 in bovine serum albumin with 0.5% ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com