Preparation method of chiral sertraline hydrochloride
A technology of sertraline hydrochloride and chirality is applied in the field of splitting of chiral sertraline hydrochloride to achieve the effects of less three wastes, high efficiency and convenient post-processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
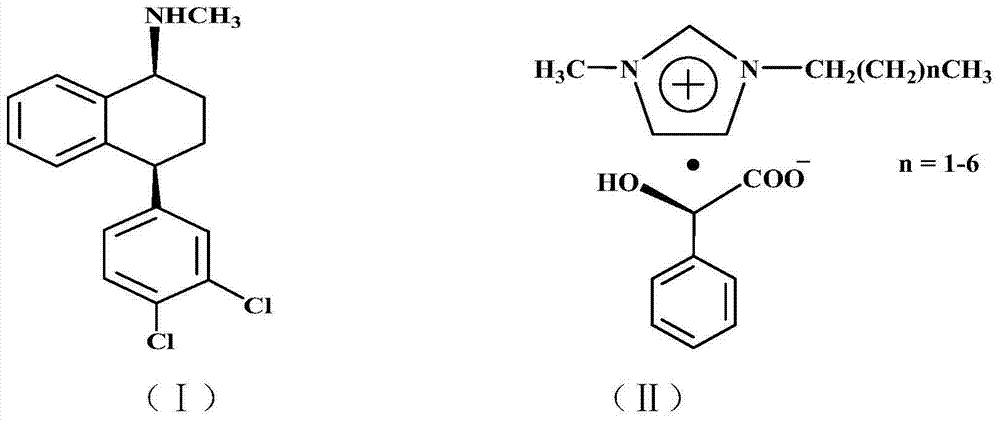
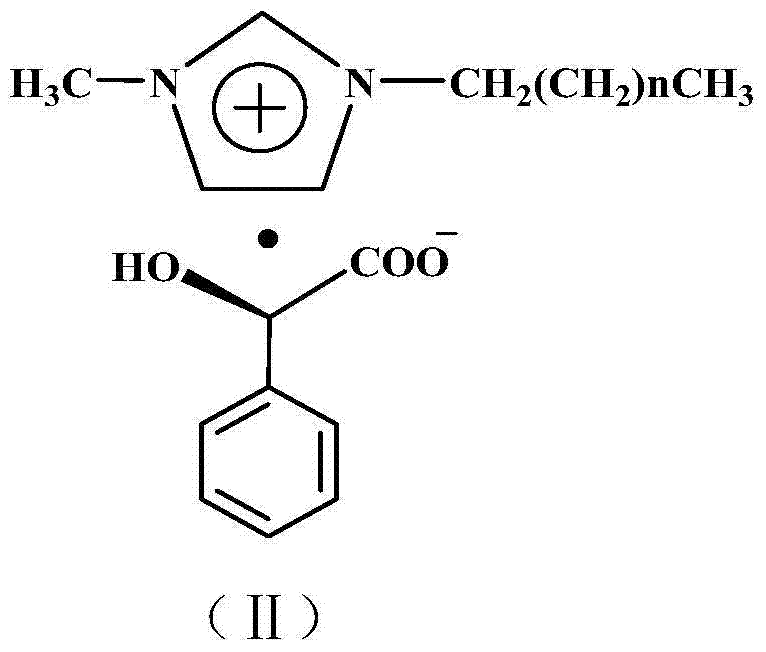
[0022] Add 306 g (1 mole) of racemic sertraline and 552 g (2 moles) of chiral D-1-methyl-3-propylimidazolium mandelate ionic liquid into a 2L three-neck flask, at 50°C After reacting for 5 hours, add 306 milliliters of absolute ethanol and stir for 1 hour, filter to obtain filter cake a and filtrate a, add 50 milliliters of 10% sodium hydroxide aqueous solution to filter cake a and stir at room temperature for 1 hour for hydrolysis, and then Add 3 × 100 ml of dichloromethane for extraction three times, combine the dichloromethane extracts and add 20% aqueous hydrochloric acid with mass concentration under stirring at room temperature until no new precipitates are formed, filter to obtain filter cake b and filtrate b, filter cake b is 137 grams of Cis-(1S,4S)-4-(3,4-dichlorophenyl)-1,2,3,4-tetrahydro-N-methyl-1-naphthylamine hydrochloride, The yield is 80%. Melting point: 248~250℃, optical purity ≥98.0%, content ≥99.0%.
Embodiment 2
[0024] Add 306 g (1 mole) of racemic sertraline and 828 g (3 moles) of chiral D-1-methyl-3-butylimidazole mandelate ionic liquid into a 2L three-neck flask, at 80°C After reacting for 3 hours, add 1530 milliliters of absolute ethanol and stir for 1 hour, filter to obtain filter cake a and filtrate a, add 50 milliliters of mass concentration 10% sodium hydroxide aqueous solution to filter cake a and stir at room temperature for 1 hour to carry out hydrolysis, then Add 3 × 100 ml of dichloromethane for extraction three times, combine the dichloromethane extracts and add 20% aqueous hydrochloric acid with mass concentration under stirring at room temperature until no new precipitates are formed, filter to obtain filter cake b and filtrate b, filter cake b is 139 grams of Cis-(1S,4S)-4-(3,4-dichlorophenyl)-1,2,3,4-tetrahydro-N-methyl-1-naphthylamine hydrochloride, Yield 81.2%. Melting point: 248~250℃, optical purity ≥98.0%, content ≥99.0%.
Embodiment 3
[0026]Add 306 grams (1 mole) of racemic sertraline and 664 grams (2 moles) of chiral D-1-methyl-3-hexylimidazole mandelate ionic liquid into a 2L three-necked flask, and react at 50°C After 5 hours, add 918 milliliters of absolute ethanol and stir for 1 hour, filter to obtain filter cake a and filtrate a, add 50 milliliters of 10% sodium hydroxide aqueous solution to filter cake a, stir at room temperature for 1 hour for hydrolysis, and then add Extract three times with 3×100 ml of dichloromethane, combine the dichloromethane extracts and add 20% hydrochloric acid aqueous solution under stirring at room temperature until no new precipitate is formed, filter to obtain filter cake b and filtrate b, filter cake b That is Cis-(1S,4S)-4-(3,4-dichlorophenyl)-1,2,3,4-tetrahydro-N-methyl-1-naphthylamine hydrochloride 138 grams, received The rate is 81%. Melting point: 248~250℃, optical purity ≥98.0%, content ≥99.0%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com