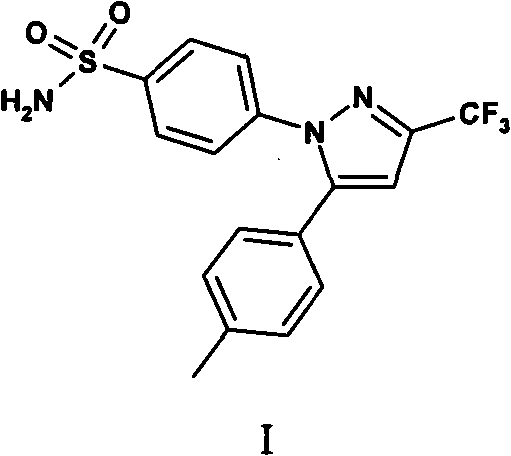
Preparation method of celecoxib
A technology of celecoxib and amides, applied in the field of new celecoxib preparation, can solve the problems of long reaction period, up to 24 hours, difficult to realize industrialization, etc., to achieve excellent product quality, reduce reaction time, avoid The effect of post-processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] Preparation of 4,4,4-trifluoro-1-(4-methylphenyl)-1,3-butanedione:
[0066] Under nitrogen protection, 2.5 L of N,N-dimethylformamide and 162.5 g (3.0 mol) of sodium methoxide were added to a 5-liter reactor, and the temperature was lowered to 5-10°C. 335.0 g (2.5 mol) of p-methylacetophenone was added dropwise for 0.5 hours. After the dropwise addition, 390.5 g (0.3 mol) of ethyl trifluoroacetate was added dropwise for 0.5 hours, and the reaction was carried out at room temperature for 0.5 hours.
[0067] Pour this solution into 7500 mL of ice water and stir mechanically. Use 37% concentrated hydrochloric acid to adjust the pH value to 3-5, stir for 20 minutes, filter with suction, and dry to obtain light yellow solid 4,4,4-trifluoro-1-(4-methylphenyl)-1,3- Diacetyl 500.6g (2.17mol), yield 86.8%.
[0068] Synthesis of Celecoxib:
[0069] Add 4 L of N, N-dimethylformamide, 534.5 g (2.39 mol) of 4-(aminosulfonyl) phenylhydrazine hydrochloride, and 90 ml of 37% concent...
Embodiment 2
[0071] One-step synthesis of celecoxib:
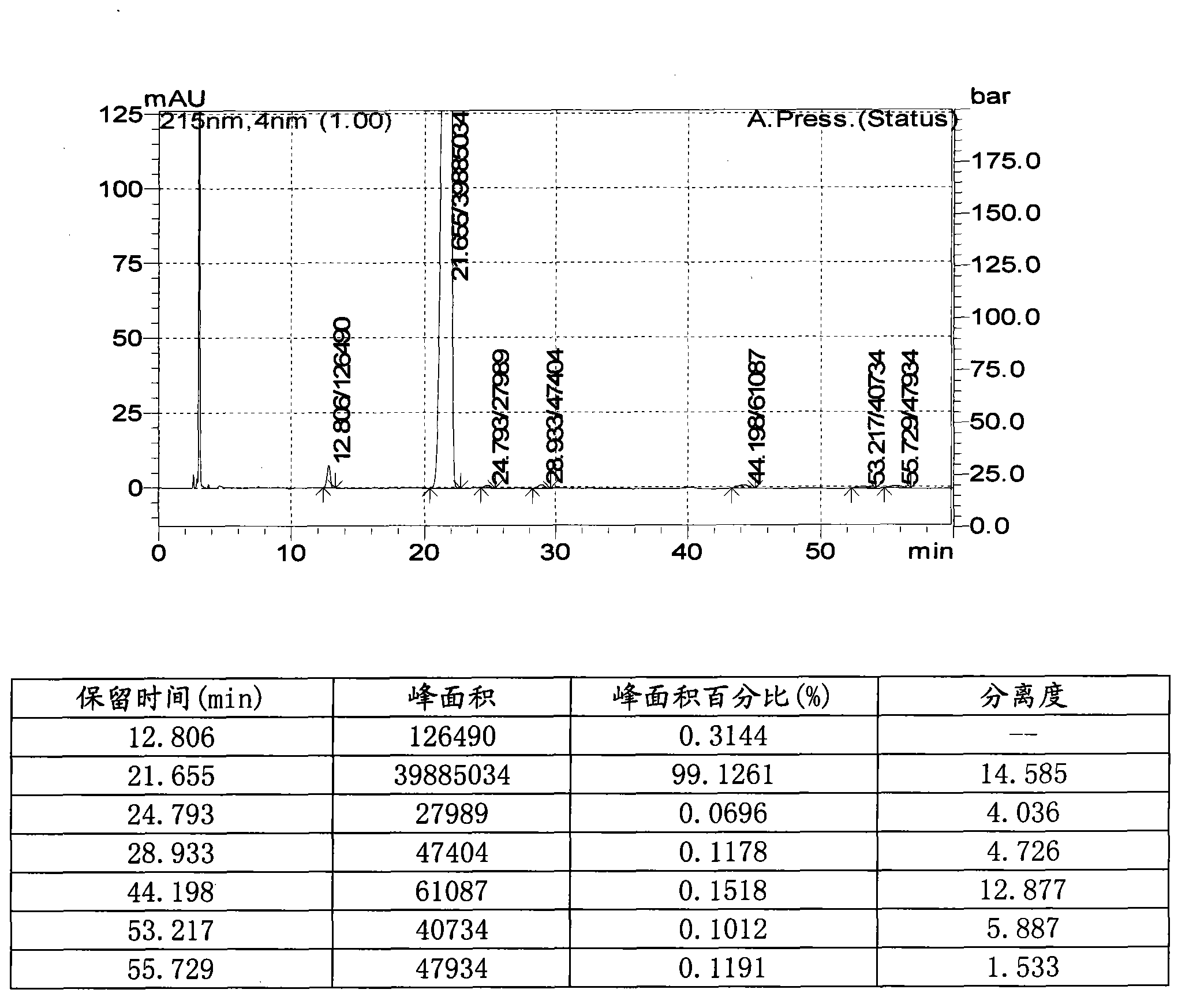
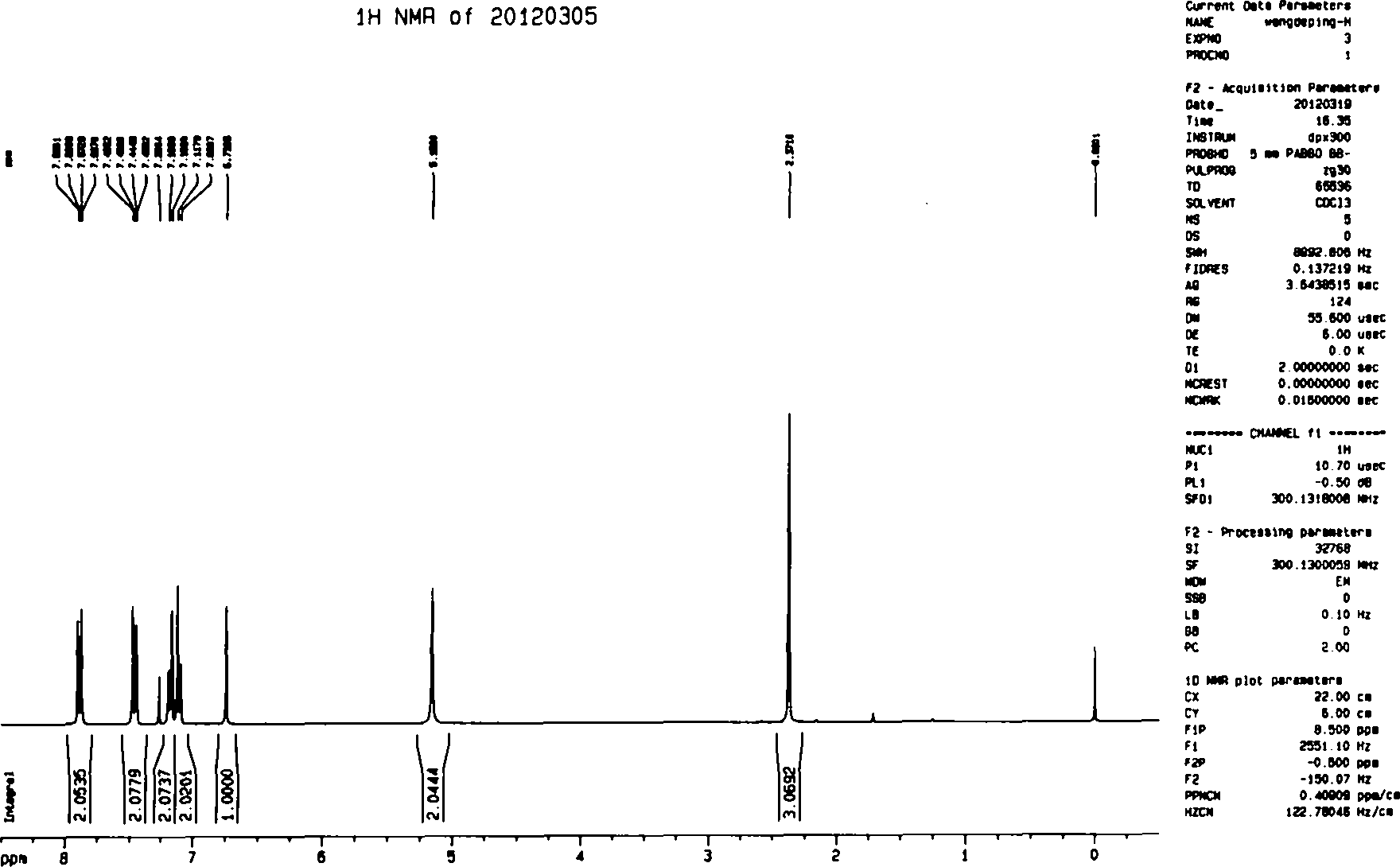
[0072] Under a nitrogen atmosphere, 7.56 kg of N,N-dimethylformamide and 331.5 g (6.13 mol) of sodium methoxide were added to a 20-liter reactor, stirred mechanically, and cooled to 0-5°C. 685.5 g (5.11 mol) of p-methylacetophenone was added dropwise for 30 minutes, and stirring was continued for 10 minutes. 798.5 g (5.62 mol) of ethyl trifluoroacetate was added dropwise over 30 minutes. Continue the reaction at room temperature for 20 minutes, slowly add 720 ml of 37% concentrated hydrochloric acid, add 1.14 kg (5.11 mol) of 4-(aminosulfonyl)phenylhydrazine hydrochloride, and heat to 55°C for 4 hours. After cooling to room temperature, 11.5 kg of water was slowly added. Stir for 1.5 hours, filter, and dry to obtain 1.57 kg (4.12 mol) of celecoxib. Yield: 80.5%, purity 99.1%, 160-164°C.
Embodiment 3
[0074] Synthesis of celecoxib in two steps using N,N-dimethylacetamide as solvent
[0075] Preparation of 4,4,4-trifluoro-1-(4-methylphenyl)-1,3-butanedione:
[0076] Under nitrogen protection, 250 mL of N,N-dimethylacetamide and 162.4 g (0.30 mol) of sodium methoxide were added to a 500 mL reaction kettle, and the temperature was lowered to 5-10°C. 335.3 g (0.25 mol) of p-methylacetophenone was added dropwise for 40 minutes. After the dropwise addition, 390.0 g (0.3 mol) of ethyl trifluoroacetate was added dropwise for 30 minutes, and the reaction was carried out at room temperature for 30 minutes.
[0077] Pour this solution into 750 mL of ice water and stir mechanically. Use 37% concentrated hydrochloric acid to adjust the pH value to 3-4, stir for 20 minutes, filter with suction, and dry to obtain light yellow solid 4,4,4-trifluoro-1-(4-methylphenyl)-1,3- Diacetyl 48.5g (0.21mol), yield 84.1%.
[0078] Synthesis of Celecoxib:
[0079] Add 400mL of N,N-dimethylacetamide...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com