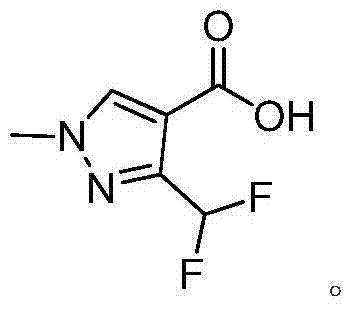
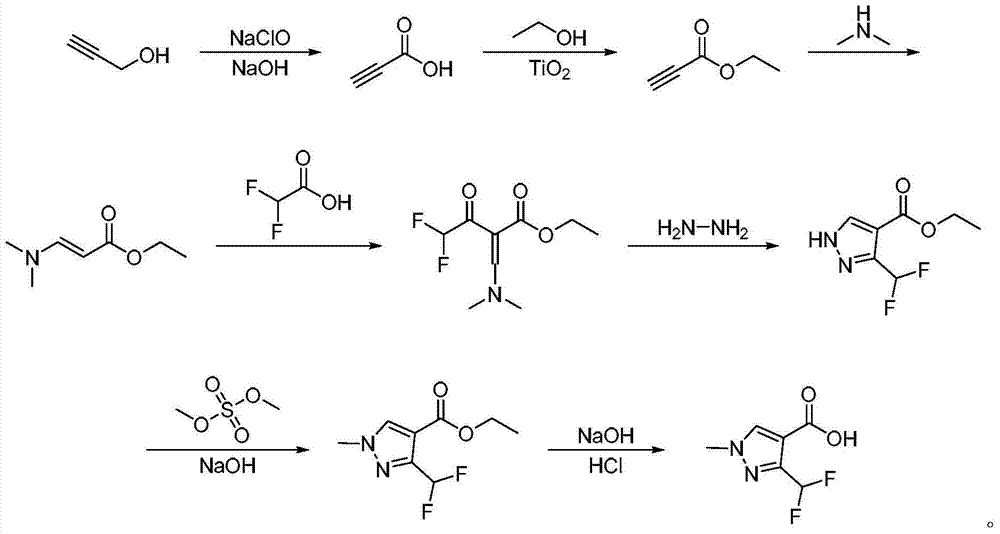
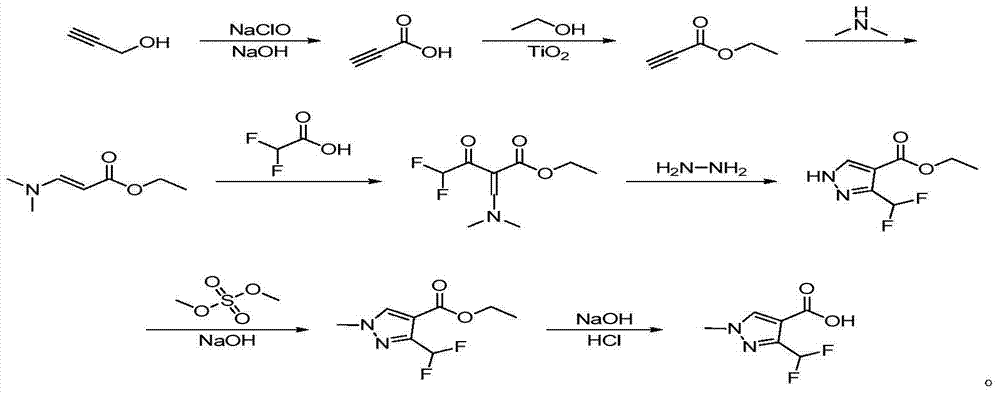
Preparation method of 3-(difluoromethyl)-1-methyl-1H-pyrazol-4-carboxylic acid
A technology of difluoromethyl and dimethylamino, applied in the field of preparation of 3--1-methyl-1H-pyrazole-4-carboxylic acid, can solve the problem of no yield report, unsuitable for industrial production, system High moisture content is required to achieve the effect of simple and safe process operation, reduced solvent recovery cost and environmental pollution risk, and high product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Add 19.6g (0.35mol) propynyl alcohol, 8.75g (0.105mol) sodium hydroxide, and 60mL water into a 1000mL three-necked flask with mechanical stirring, control the reaction temperature at -5 to 5°C with ice-salt water, and dissolve 408mL (0.98 mol) 2.4mol / L sodium hypochlorite solution was added dropwise into the reaction bottle within 1 hour, and the reaction was continued for 1 hour. After the reaction, adjust the pH to 1 with concentrated hydrochloric acid and then stir for one hour, extract with ethyl acetate, stand still and separate the phases, and distill the organic phase under reduced pressure to obtain 22.4 g of propiolic acid with a content of 98.7% (liquid chromatography). The rate is 90.2%.
[0031] Add 22.4g (0.316mol) propiolic acid, 0.067g (0.3% of the mass of propiolic acid) nano-scale titanium dioxide, 17.4g (0.379mol) ethanol, and 130mL toluene to the tank with mechanical stirring, condenser and water separator A 500mL three-necked flask was refluxed and ...
Embodiment 2
[0038] Add 19.6g (0.35mol) propynyl alcohol, 8.75g (0.105mol) sodium hydroxide, and 80mL water into a 1000mL three-neck flask with mechanical stirring, and control the reaction temperature at -5 to 5°C with ice-salt water, and dissolve 408mL (0.98 mol) 2.4mol / L sodium hypochlorite solution was added dropwise into the reaction bottle within 1 hour, and the reaction was continued for 1 hour. After the reaction, adjust the pH to 1 with concentrated hydrochloric acid, then stir for one hour, extract with ethyl acetate, stand still and separate the phases, and distill the organic phase under reduced pressure to obtain 22.7 g of propiolic acid with a content of 97.9% (liquid chromatography). The rate is 90.8%.
[0039] Add 22.7g (0.318mol) propiolic acid, 0.091g (0.4% of the mass of propiolic acid) nanoscale titanium dioxide, 17.5g (0.38mol) ethanol, and 130mL toluene into a 500mL vessel with mechanical stirring, condenser and water separator The three-necked flask was refluxed and...
Embodiment 3
[0046] Add 19.6g (0.35mol) propynyl alcohol, 8.75g (0.105mol) sodium hydroxide, and 80mL water into a 1000mL three-neck flask with mechanical stirring, and control the reaction temperature at -5 to 5°C with ice-salt water, and dissolve 408mL (0.98 mol) 2.4mol / L sodium hypochlorite solution was added dropwise into the reaction bottle within 1 hour, and the reaction was continued for 1 hour. After the reaction, adjust the pH to 1 with concentrated hydrochloric acid and then stir for one hour, extract with ethyl acetate, stand and separate the phases, and distill the organic phase under reduced pressure to obtain 22.7 g of propiolic acid with a content of 98.3% (liquid chromatography). The rate is 90.9%.
[0047] Add 22.7g (0.318mol) propiolic acid, 0.091g (0.4% of the mass of propiolic acid) nanoscale titanium dioxide, 17.6g (0.382mol) ethanol, and 160mL toluene into a 500mL vessel with mechanical stirring, condenser and water separator The three-necked flask was refluxed and w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com