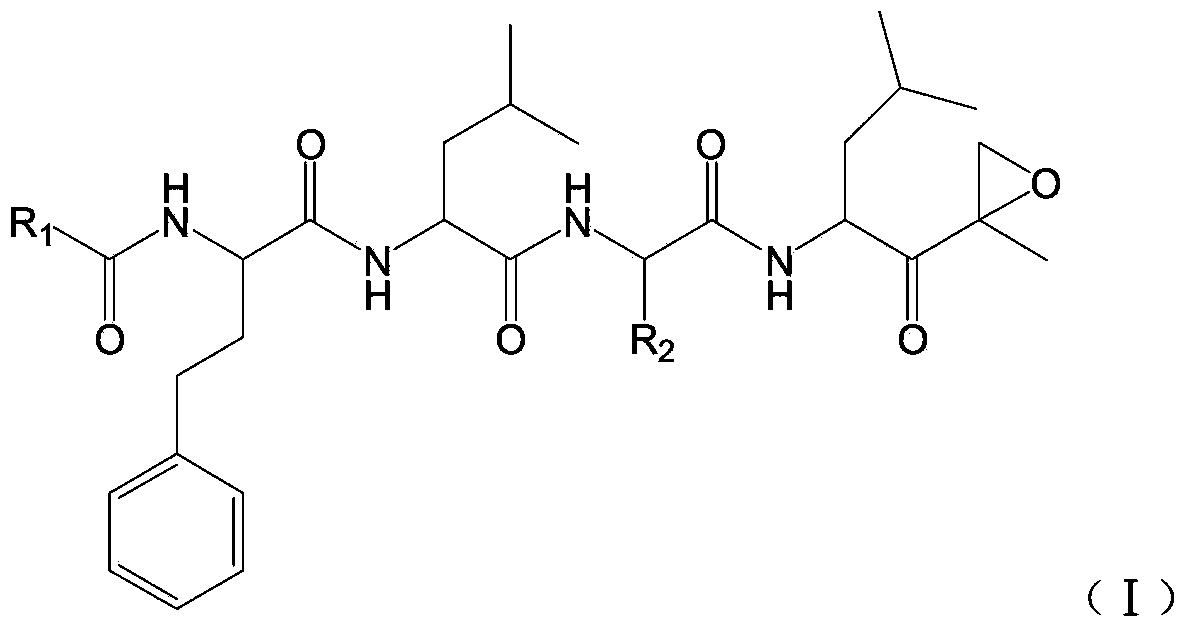
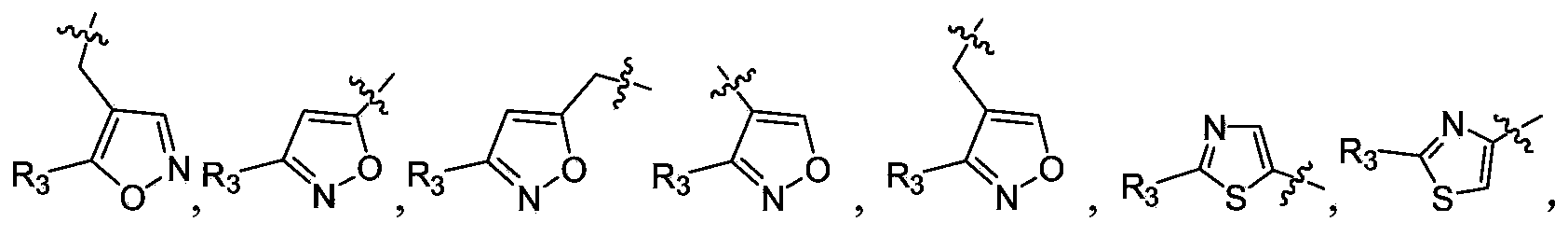
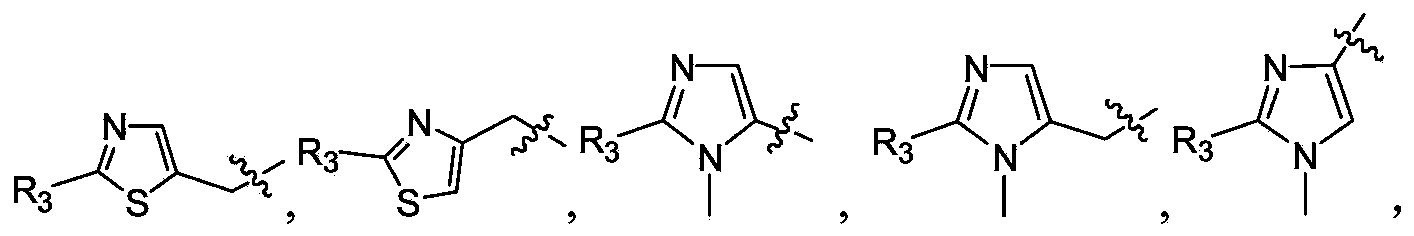
Polypeptide epoxy ketone compound
A compound and solvate technology, applied in the direction of peptides, drug combinations, peptide/protein components, etc., can solve problems such as inability to inhibit T-L activity, PGPH activity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0210] Example 1: Determination of Inhibition of 20S Proteasome CT-L, T-L, and PGPH Activities in Vitro.
[0211]The purified human 20S proteasome was used for in vitro determination of CT-L, T-L and PGPH activities. The concentrations of 20S proteasome were 2, 4 and 8 nmol / L, respectively, and succinyl-Leu-Leu-Val-Tyr-AMC ( 10 μmol / L), Z-leu-Leu-Glu-AMC (10 μmol / L) and Boc-Leu-Arg-Arg-AMC (50 μmol / L) were used as substrates, and the reaction contained 0.5mM ethylenediaminetetraacetic acid, 0.001% sodium dodecyl sulfate (SDS) and 0.05% NP-40 in 20mM triisopropylethanesulfonyl buffer solution (pH8.0). Stock solutions of proteasome inhibitors were prepared in dimethylsulfoxide (DMSO), the final concentration of DMSO in the test mixture was 1%. The reaction was carried out at a temperature of 27°C. Proteasome activity was determined by detecting the cleaved 7-amino-4-methylcoumarin fluorophore with a fluorophotometer. IC 50 (Half inhibitory concentration) values were determ...
Embodiment 2
[0212] Example 2: Determination of the inhibitory effect on CT-L, T-L and PGPH activities of 20S proteasome in blood.
[0213] Take 800 microliters of fresh whole blood samples from CD-1 mice by cardiac puncture into test tubes containing sodium heparin, centrifuge at 150xg for 5 minutes at 4°C, and wash the centrifuged precipitate with ice-cold phosphate-buffered saline three times. Each time the pellet was redissolved in 1 mL of cold phosphate-buffered saline and centrifuged at 6000 x g for 10 min at 4 °C. After the last wash, 200 μL of dissociation solution (phosphate-buffered saline containing 5 mM EDTA, pH 8.0) was added, kept for 1 hour, and the cells were dissociated, and then centrifuged at 6000×g for 10 minutes at 4°C. The protein concentration in the above-mentioned blood lysate was determined by the bicincholic acid method (BCA). Add about 100 μ g protein to HEPES buffer solution (pH7.5); Add 0.2, 1 or 5 μ M of the compound of the present invention to the above mi...
Embodiment 3
[0214] Example 3: Determination of Inhibition of CT-L, T-L and PGPH Activities of 20S Proteasome in Vivo.
[0215] In vivo proteasome activity assays were performed in BALB / c nude mice. Take the polypeptide epoxy ketone compound described in the present invention as the test article and administer it to mice (every group of 5-6 mice) by intravenous injection in a tolerable dose, and the test article is prepared in a mixture containing 10-20 %(w / v) hydroxypropyl-B-cyclodextrin, 2.5% DMSO and 10mM sodium citrate in the vehicle (pH3.0-3.5). One hour after the injection, a whole blood sample was collected by cardiac puncture into a test tube containing sodium heparin and centrifuged at 150xg for 5 minutes at 4°C. The centrifuged pellet was washed three times with ice-cold phosphate-buffered saline. Each time the pellet was redissolved in 1 mL of cold phosphate-buffered saline and centrifuged at 6000 x g for 10 min at 4 °C. After the last wash, 200 μL of dissociation solution (p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com