Sheet and liquid combination systems for dermal drug delivery
A thin-layer, medium-liquid technology, applied in nervous system diseases, antipyretics, drug combinations, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
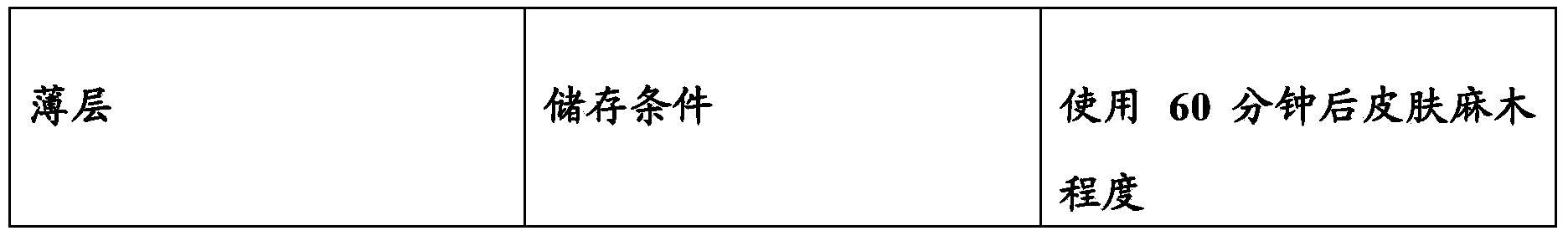
[0073] The following system containing a thin layer and a vehicle fluid that can provide skin anesthesia or analgesia is an example of an embodiment of the present invention.
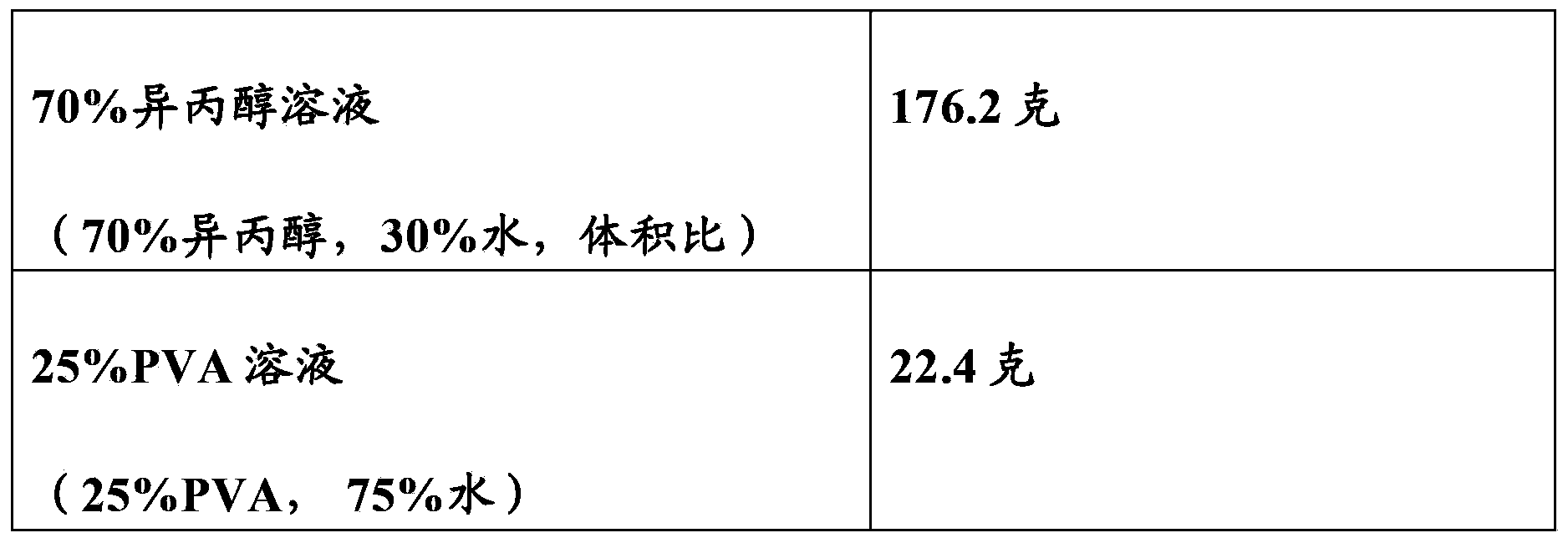
[0074] (1) In this and other examples, tetracaine or TC refers to tetracaine base unless otherwise stated. Tetracaine (base) (purchased from Spectrum Chemical) of the United States Pharmacopoeia specification was dissolved in a 70:30 volume ratio isopropanol aqueous solution (Western Family brand) to obtain a 10% (weight ratio) tetracaine solution. In this and other examples, "70% isopropanol" or "70% isopropanol solution" or "rubbing alcohol" refers to Western Family brand rubbing alcohol (70% isopropanol, 30% water, volume ratio) (2) Laminate a layer of gauze (Dusoft brand non-woven fabric, Dumex, No. 84122, single layer) to a layer of polyurethane film (Tegaderm film from 3M, purchased from Ortho-Med) to form a composite Thin layer. (3) Distribute 0.72 g of 10% tetracaine solution evenly on the gauze ...
example 2
[0079] In order to obtain skin anesthesia or analgesia, the inventors created a system containing a thin layer and a medium liquid as described below, and used the system as an exemplary embodiment of the present invention.
[0080] Step 1: Heat 10 grams of polyvinyl alcohol (PVA, sample from Amresco, with a molecular weight of 30,000 to 50,000) and 90 grams of distilled water to about 70°C and stir until a uniform 10% (weight ratio) PVA solution is obtained.
[0081] Step 2: Add 5 grams of the 10% PVA solution obtained in Step 1 to 6 grams of rubbing alcohol. Shake the container until a homogeneous solution is obtained.
[0082] Step 3: Add 0.58 g of tetracaine base (U.S. Pharmacopoeia specification, purchased from Spectrum Chemical Company) to the solution of step 2. Shake the container until all the tetracaine particles are dissolved to obtain a solution containing 5% tetracaine and 4.3% PVA.
[0083] Step 4: Hang a piece of gauze (Non-Woven Sponges from Dusoft, product number 841...
example 4
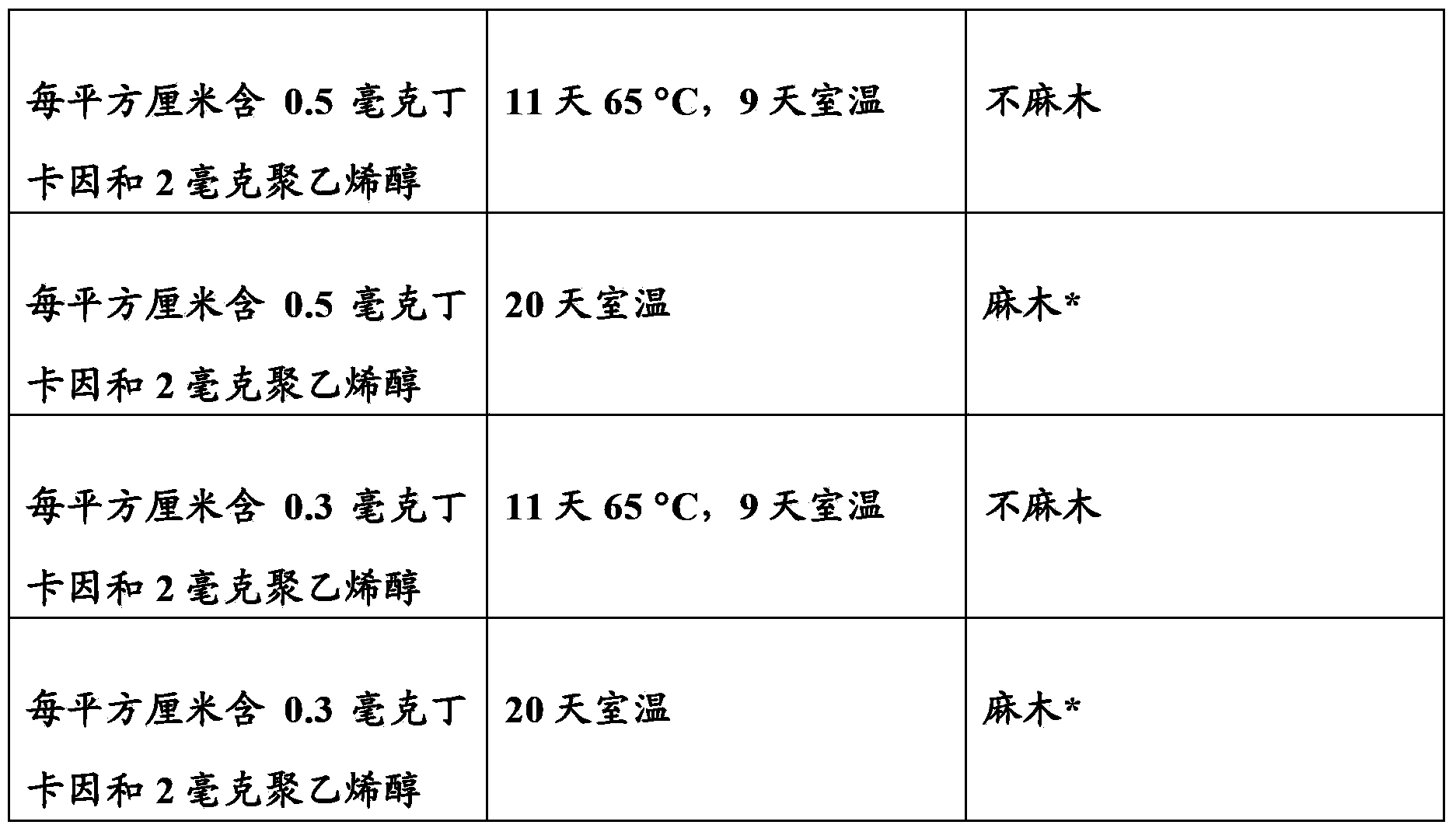
[0102] Mix 3.04 grams of the 25% PVA solution in step 1 of Example 3, 4.55 grams of rubbing alcohol, and 0.51 grams of tetracaine base until a transparent solution containing 6.3% tetracaine and 9.4% VPA is obtained. Spread about 6.7 grams (about 7 ml) of this solution evenly on a 190 square cm gauze and 3M9832 composite film (same as the composite film in step 3 of Example 3), and dry it in the oven. The dried thin layer contains 2.2 mg tetracaine and 3.3 mg PVA per square centimeter.
[0103] The following experiment uses distilled water as the medium liquid to test the abrasion resistance and anesthesia effect of the above system.
[0104] Spray distilled water with a spray bottle onto the left forearm of a human subject. The water droplets of distilled water densely covered the skin area, but did not form a continuous water layer.
[0105] Place a 2 x 3 cm thin layer (containing 2.2 mg of tetracaine and 3.3 mg of PVA per square cm) on the vehicle liquid already on the skin, wit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com