Method for recycling CO to produce nitrogen oxides in oxalate tail gas
A technology for producing nitrogen oxides and oxalate esters, applied in chemical instruments and methods, separation methods, dispersed particle separation, etc., can solve the problems of unrecycled use, etc., and achieve the effects of protecting the environment, reducing emissions, and saving costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
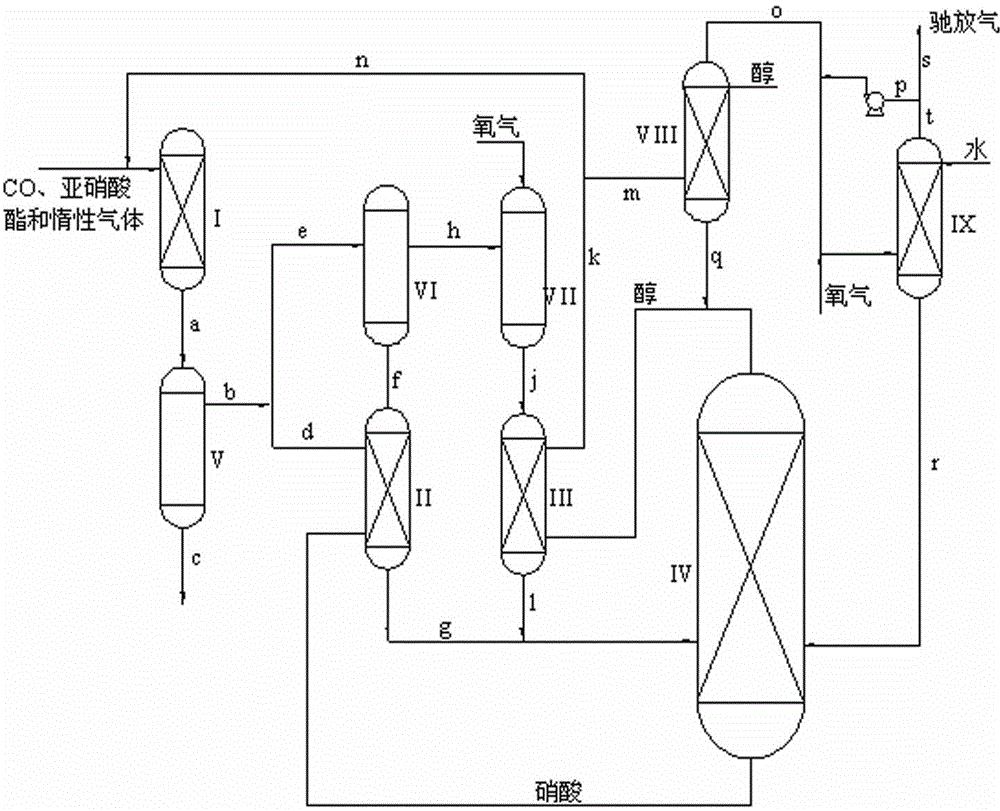
[0036] The tail gas discharged from the top of the regeneration reactor contains 12% by volume of ethyl nitrite, 4% by volume of nitrogen monoxide, 19% by volume of carbon monoxide, and 65% by volume of nitrogen. Spray ethanol, the weight ratio of ethanol to ethyl nitrite is 3: 1, the operating temperature is 20°C, the operating pressure is 5.0 Mpa, and the liquid in the alkyl alcohol washing tower tank is discharged into the regeneration reactor of CO oxalate ester production , the gas at the top of the alkyl alcohol scrubber reacts with oxygen to oxidize NO in nitrogen oxides to NO 2 , and then enter the water scrubber to contact with water, the liquid in the water scrubber tank returns to the nitric acid concentration tower; part of the gas at the top of the water scrubber is mixed with the gas at the top of the alkyl alcohol scrubber, and then mixed with oxygen Contact, control of NO and O in the mixed gas 2 The volume ratio of the nitrogen oxides is 1:4, and the other pa...
Embodiment 2
[0038] The tail gas discharged from the top of the regeneration reactor contains 6% methyl nitrite, 8% nitrogen monoxide, 25% carbon monoxide, and 61% nitrogen by volume percentage. The upper part of the tower is sprayed with methanol, the weight ratio of methanol to methyl nitrite is 10:1, the operating temperature is 20°C, and the operating pressure is 0.1 MPa. The liquid in the tower tank of the alkyl alcohol washing tower is discharged into the regeneration of oxalate Reactor, the gas at the top of the alkyl alcohol scrubber reacts with oxygen to oxidize NO in nitrogen oxides to NO 2 , and then enter the water scrubber to contact with water, the liquid in the water scrubber tank returns to the nitric acid concentration tower; part of the gas at the top of the water scrubber is mixed with the gas at the top of the alkyl alcohol scrubber, and then mixed with oxygen Contact, control of NO and O in the mixed gas 2 The volume ratio of the nitrogen oxides is 2:1, and the other ...
Embodiment 3
[0040] The tail gas discharged from the top of the regeneration reactor contains 11% by volume of n-butyl nitrite, 10% by volume of nitrogen monoxide, 24% by volume of carbon monoxide, and 55% by volume of nitrogen. It enters from the bottom of the alkyl alcohol scrubber, and the alkyl alcohol scrubber The upper part is sprayed with n-butanol, the weight ratio of n-butanol to n-butyl nitrite is 8:1, the operating temperature is 50°C, and the operating pressure is 3.0 MPa. Ester regeneration reactor, the gas at the top of the alkyl alcohol scrubber reacts with oxygen to oxidize NO in nitrogen oxides to NO 2 , and then enter the water scrubber to contact with water, the liquid in the water scrubber tank returns to the nitric acid concentration tower; part of the gas at the top of the water scrubber is mixed with the gas at the top of the alkyl alcohol scrubber, and then mixed with oxygen Contact, control of NO and O in the mixed gas 2 The volume ratio of the nitrogen oxides is ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com