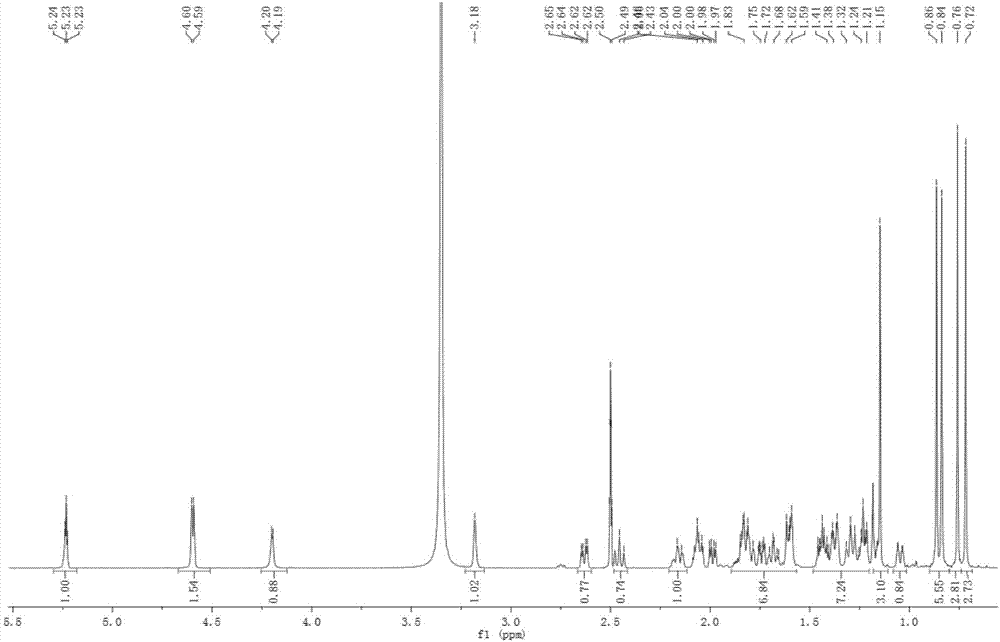
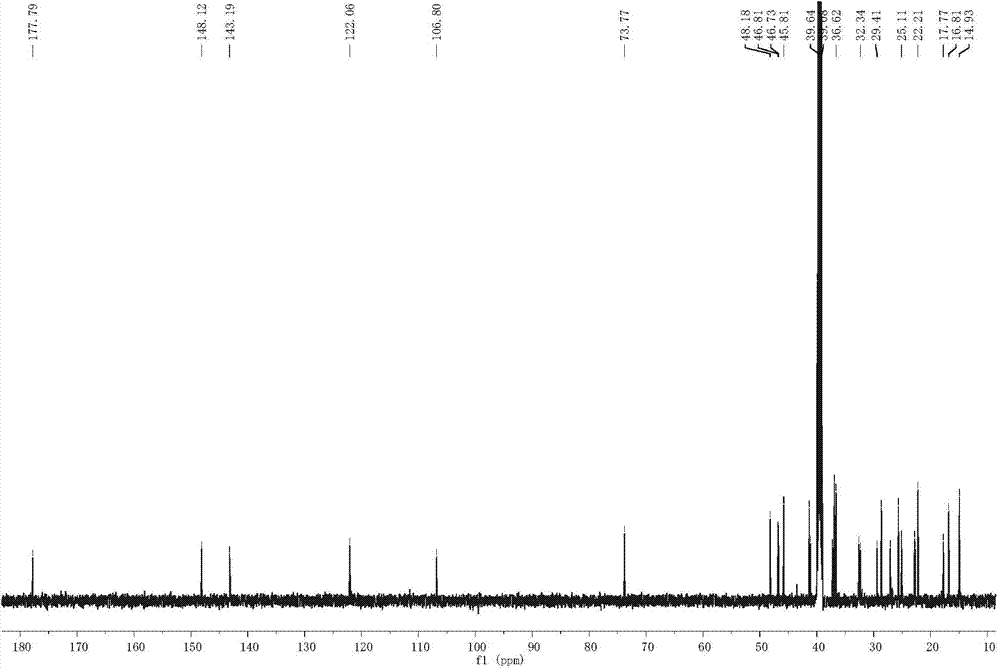
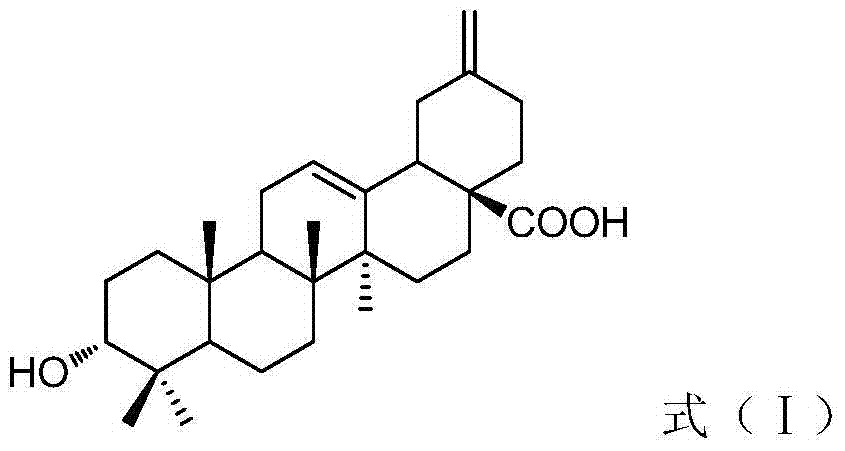
Preparation method of 3(alpha)-hydroxyl-30-olive-12,20(29)-diene-28-acid and application in preparing anti-tumor drug
An anti-tumor drug and olean fruit technology is applied in the field of biomedicine to achieve the effects of rich material sources, good application development potential and good environmental benefits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1: Preparation of 3α-hydroxy-30-olean-12,20(29)-diene-28-acid in Akebia clover fruit
[0025] 1.1 Plant source and identification
[0026] The fruit samples of Akebia trifolia (Thumb.) Koidz., a plant material for extraction, were collected from Hunan Province in September 2009 and identified by Xing Fuwu, a researcher at South China Botanical Garden, Chinese Academy of Sciences.
[0027] 1.2 Extraction and separation
[0028] The sample (dried fruit of Akebia trifoliata, weighing 1.0 kg) was crushed and extracted three times with 95% ethanol at room temperature, the combined filtrate was concentrated under reduced pressure to remove the organic solvent ethanol, and the crude extract of the total extract was obtained. The total extract was suspended in 500ml of water, extracted three times with an equal volume of petroleum ether, and the extract was concentrated under reduced pressure to obtain total petroleum ether extract (16g). Dissolve the petroleum ether ...
Embodiment 2
[0033] Example 2: Preparation of 3α-hydroxy-30-olean-12,20(29)-diene-28-acid in the stems and leaves of Akebia trifoliata
[0034] 2.1 Plant source and identification: same as Example 1
[0035] 2.2 Extraction and separation:
[0036] The sample (Akebia trifoliate stems and leaves, dry weight 1.0 kg) was crushed and extracted three times with 95% ethanol at room temperature, and the combined filtrate was concentrated under reduced pressure to remove the organic solvent ethanol to obtain the crude extract of the total extract. Suspend the total extract in 500ml of water, extract three times with an equal volume of petroleum ether, and concentrate the extract under reduced pressure to obtain total petroleum ether extract (13g). Dissolve the petroleum ether total extract with acetone (150mL), add normal phase silica gel (80-100 mesh) to mix the sample at a weight ratio of 1:1.5, evaporate to dryness, and dry the column (200-300 mesh, 300g) to dry Samples were loaded using petro...
Embodiment 3
[0038] Take the stems, leaves or fruits of Akebia, Akebia changxu, Akebia basilicate and Akebia trilobata as samples, and finally purify according to the extraction and separation methods described in Example 2 to obtain the pure compound 3α-hydroxyl of formula (I) -30-Olean-12,20(29)-dien-28-oic acid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com