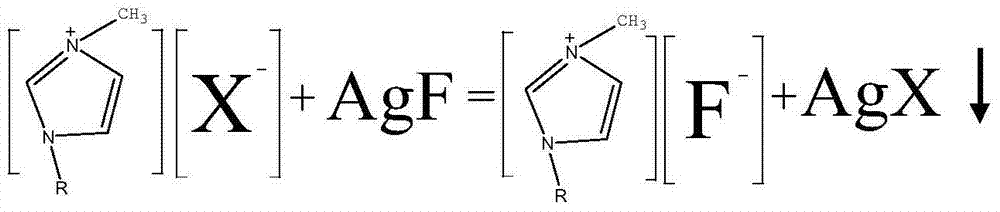
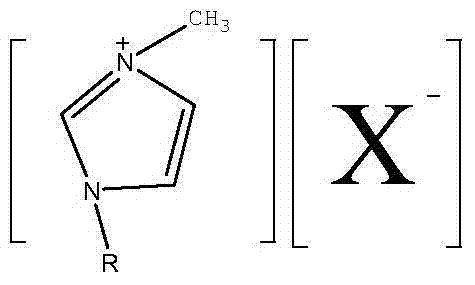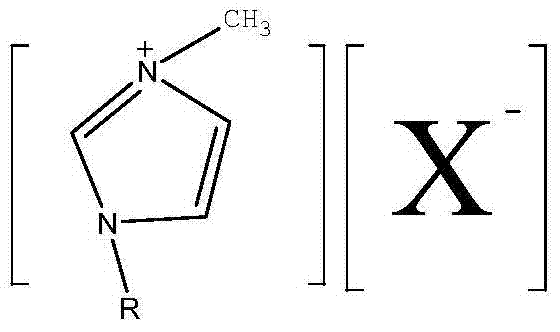Synthesis method of disubstituted-fluoride imidazole ionic liquid
A technology of imidazole fluoride and ionic liquid, which is applied in the field of ionic liquid synthesis, can solve problems such as complicated operation, difficult to remove impurities, and strict equipment requirements, and achieve the effect of simple operation and fast reaction speed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] 1, the synthetic method of 3-dimethyl imidazole fluoride, its steps are:
[0035] 1) Take 10g of 1,3-dimethyl imidazole chloride (purity 90%) and 10.44g AgF (purity 90%) respectively, and dissolve them in 25ml and 20ml of distilled water respectively. After the solution is mixed, a metathesis reaction occurs rapidly to produce a white precipitate. Stir until Fully react; after the stirring is stopped, the precipitate settles rapidly, and the supernatant is a clear liquid;
[0036]2) Prepare 0.01ml / L 1,3-dimethylimidazolium chloride solution, add it into the supernatant by titration method, there will be a white precipitate, and there will be no precipitate after adding a total of 5.5ml solution;
[0037] 3) Filtrate to obtain the filtrate and filter residue; the filtrate was left to stand for 6 hours, and the filter residue was fully dried and weighed, the weight was 11.67g, number 1;
[0038] 4) Take a small glass bottle, take 1 drop of standing solution, add a small ...
Embodiment 2
[0042] 1, the synthetic method of 3-dimethyl imidazole fluoride, its steps are:
[0043] 1) Dissolve 7.63g of AgF (purity 95%) in 12ml of distilled water and mix it with 10g of 1,3-dimethylimidazole bromide (purity 99%) dissolved in 12.5ml. After the solution is mixed, metathesis reaction occurs rapidly to produce yellow precipitate. Stir until fully reacted; after the stirring stops, the precipitate settles rapidly, and the supernatant is a clear liquid;
[0044] 2) Prepare 0.01ml / L 1,3-dimethylimidazole bromide solution, add it to the supernatant by titration method, there will be a light yellow precipitate, and there will be no precipitate after adding 1ml of the solution in total;
[0045] 3) Filtrate to obtain filtrate and filter residue. Weigh the filter residue after fully drying, the weight is 11.01g, number 1;
[0046] 4) Take a small glass bottle, take 1 drop of filtrate, add a small amount of distilled water to dilute, add KCl, and check whether there is still Ag ...
Embodiment 3
[0050] 1-ethyl-3-methyl imidazole fluoride synthetic method, its steps are:
[0051] 1) Take 10g of 1-ethyl-3-methylimidazole chloride (purity 99%) and 8.8gAgF (purity 98%), respectively, and dissolve them in 25ml and 20ml of distilled water respectively. After the solutions are mixed, metathesis reaction occurs rapidly to produce white precipitate. Stir with a magnetic stirrer for 24 hours to fully react; after the stirring stops, the precipitate settles rapidly, and the supernatant is a clear liquid;
[0052] 2) Prepare 0.01ml / L of 1-ethyl-3-methylimidazole chloride solution, add it to the supernatant by titration method, there will be a white precipitate, and there will be no precipitate after adding a total of 32ml of the solution;
[0053] 3) Filtrate to obtain filtrate and filter residue. The filtrate was left to stand for 24 hours, and the filter residue was fully dried and weighed, the weight was 9.97g, and the number 1;
[0054] 4) Take a small glass bottle, take 1 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com