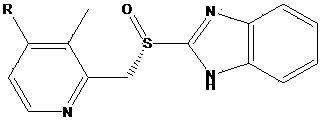
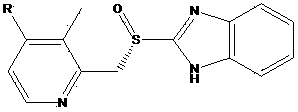
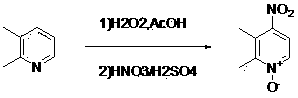Preparation method for Dexiansoprazole
A technology of dexlansoprazole and nitro, which is applied in the field of preparing dexlansoprazole, can solve the problems that sulfone is difficult to remove, and achieve the effects of being beneficial to scale-up production, easy to obtain, and simple to operate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0020] The preparation of 2,3-dimethyl-4-nitropyridine-N-oxide, its structural formula is as follows:
[0021]
[0022] Add 10.7 g (0.1 mol) of 2,3-lutidine, 20 mL of glacial acetic acid, and 16 mL (0.15 mol) of hydrogen peroxide with a mass fraction of 30% into a 250 mL three-necked bottle, and heat to 50 ℃, keep warm for 12 hours, distill out water and acetic acid, add dropwise the mixed acid solution prepared by 30 mL (0.54mol) concentrated sulfuric acid and 28 mL (0.4mol) concentrated nitric acid, drop it for about 30 minutes, react at 120~125℃ for 2 hours, cool to room temperature. Pour into 200 mL of cold water, neutralize to PH10~12 with 5mol / L sodium hydroxide solution, extract with dichloromethane (60mL×3), dry over anhydrous sodium sulfate, filter, and concentrate to obtain a yellow solid, which is washed with acetic acid The ethyl ester was recrystallized to obtain 12.5 g of 2,3-dimethyl-4-nitropyridine-N-oxide. Yield 78.1%.
[0023] The preparation of 2-aceto...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com