Preparation method of fluorene compounds
A fluorene compound and compound technology are applied in the field of preparation of fluorene compounds, and can solve the problems of harsh gas-phase reaction reaction conditions, difficult industrial synthesis, complex reaction types and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~9
[0079] Preparation of raw materials biphenyltriazene 1b-1i and 1aa: In a 50ml Schlenck tube, add S1 (4.0mmol, 1.0equiv), o-methylphenylboronic acid (4.8mmol, 1.2equiv, CAS: 16419-60-6 ),Pd(PPh 3 ) 4 (0.12mmol, 0.03equiv) and Cs 2 CO 3 (10mmol, 2.5 equiv). Add dioxane / H 2 O (5 / 1, 24ml), and cover the lid immediately after replacing the air with nitrogen. After stirring at 100°C for 12 hours, it was detected that the reaction of the raw materials was complete. After cooling, water (20 mL) was added to quench the reaction, and the aqueous layer was extracted twice with ethyl acetate. The combined organic layers were washed with saturated NaCl and dried over anhydrous sodium sulfate. The solvent was spun off, and the biphenyltriazenes 1b-1i and 1aa were obtained by passing through the column. The reaction formula is as follows:
[0080]
[0081] The yield of the biphenyltriazene 1b-1i of embodiment 1~9 and 1aa
[0082]
[0083] The structure and characterization da...
Embodiment 10~14
[0103] Preparation of raw materials biphenyltriazene 1j, 1l, 1o, 1s and 1v: In a 50ml Schlenck tube, add triazene phenylboronic acid (4.0mmol, 1.0equiv, CAS: 869670-79-1), o-iodotoluene S2 (4.8mmol, 1.2equiv), Pd (PPh 3 ) 4 (0.12mmol, 0.03equiv) and Cs 2 CO 3 (10mmol, 2.5 equiv). Add solvent dioxane / H 2 O (5 / 1, 24ml), and cover the lid immediately after purging the air with nitrogen. After stirring at 100°C for 12 hours, it was detected that the reaction of the raw materials was complete. After cooling, water (20 mL) was added to quench the reaction, and the aqueous layer was extracted twice with ethyl acetate. The combined organic layers were washed with saturated NaCl and dried over anhydrous sodium sulfate. Rotate off the organic solvent, and pass through the column to obtain biphenyltriazene. The reaction formula is as follows:
[0104]
[0105] The yield of the biphenyltriazene 1j, 1l, 1o, 1s and 1v of embodiment 10~14
[0106]
[0107]
[0108]
[0...
Embodiment 15~28
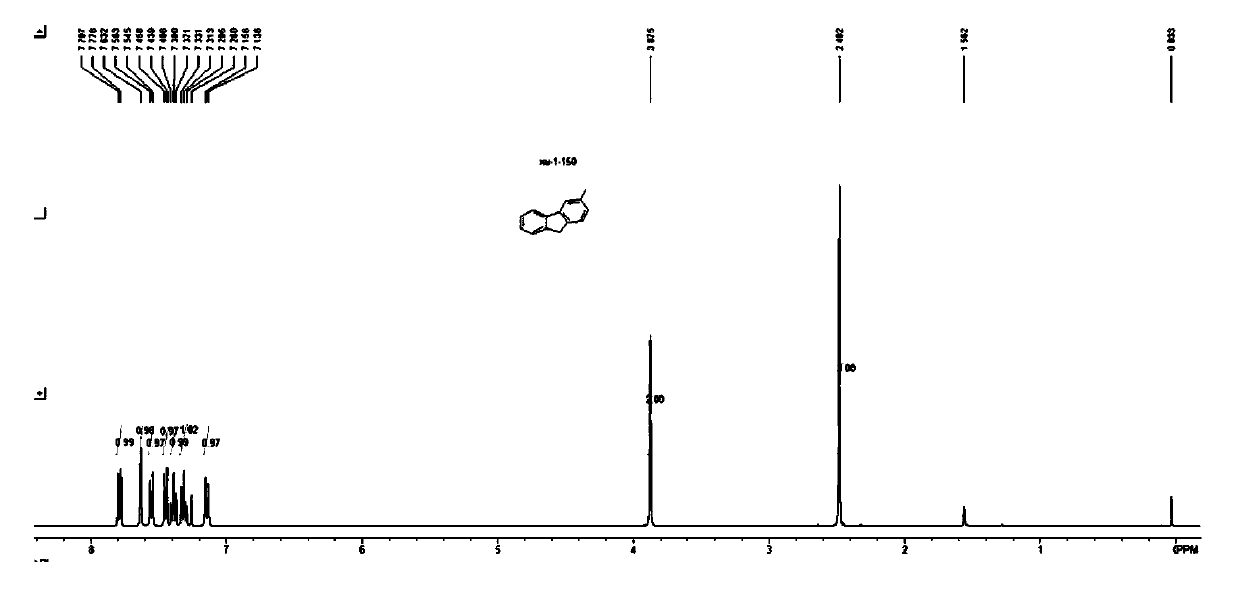
[0119] The preparation of fluorene compound 2: at room temperature, put 1mmol biphenyltriazene in 5mL toluene, add CF dropwise under stirring 3 COOH (CAS: 76-05-1) 456 mg (4 mmol). After stirring for 1 h at 100°C, it was detected that the reaction of the raw materials was complete. Add NaHCO after cooling 3 The reaction was quenched with aqueous solution (10 ml, 0.6 mol / L), and the aqueous layer was extracted 3 times with ethyl acetate. The combined organic layers were washed with saturated NaCl and dried over anhydrous sodium sulfate. The organic solvent was spun off, and the fluorene compound 2 was obtained by passing through the column. The reaction formula is as follows
[0120]
[0121] The yield of the fluorene compound 2 that embodiment 15~28 obtains
[0122]
[0123]
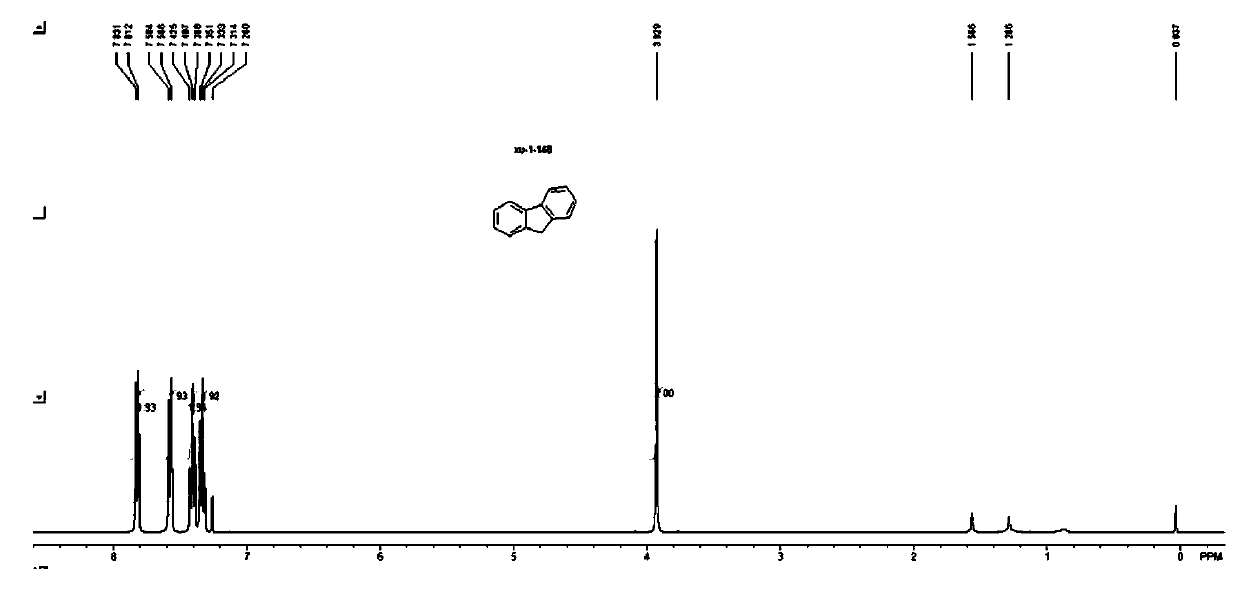
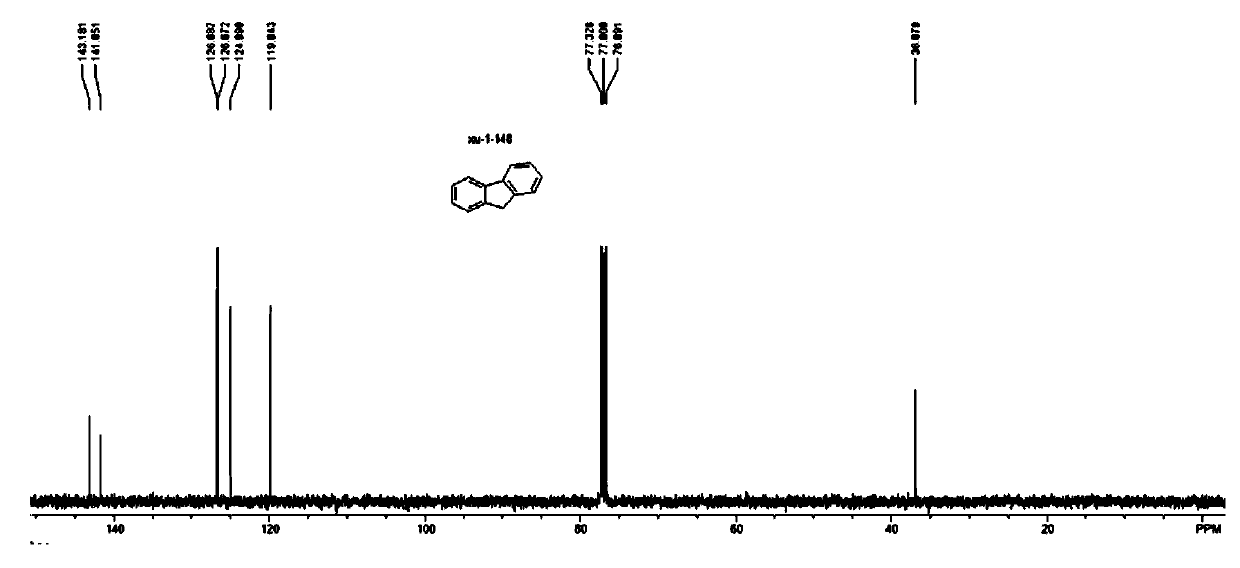
[0124] The characterization data of the product of fluorene compound 2b (CAS: 86-73-7) prepared in Example 15 are as follows:
[0125]
[0126] 2b: white solid; melting point: 106-107°C...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com