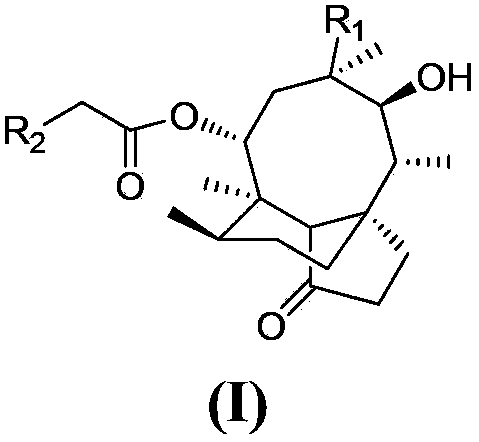
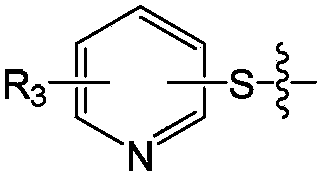
A class of pleuromytilin derivatives, drug composition, synthesis methods and uses thereof
A technology for pleuromutilin and compounds, applied in the fields of pharmacy, drug synthesis and pharmacology, can solve the problem of low activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0088] Example 1 14-O-[(5-aminomethylpyridine)-3-thioacetyl]-mutriptyline
[0089] (a) Methyl 5-bromonicotinate
[0090]
[0091]Dissolve 5-bromonicotinic acid (3.0g, 15mmol) in anhydrous methanol (20ml), add 98% concentrated sulfuric acid (4ml) dropwise at room temperature, stir for 10min after addition, heat up to reflux for 15h, distill methanol off, add water to dilute , add saturated aqueous sodium bicarbonate solution, adjust the pH to neutral, extract with ethyl acetate, wash with water, dry over anhydrous sodium sulfate, filter, evaporate the solvent, and the residue is subjected to silica gel column chromatography to obtain white flaky crystals (2.6g, 80%).
[0092] 1 H NMR (300MHz, CDCl 3 )δ9.13(s,1H),8.85(s,1H),8.45(s,1H),3.97(s,3H)MS(EI)m / z:216(M+H) + .
[0093] (b) 3-bromo-5-hydroxymethylpyridine
[0094]
[0095] Dissolve 5-bromo-nicotinic acid methyl ester (3g, 13.1mmol) in absolute ethanol (50ml), add sodium borohydride (1.48g, 39.1mmol) in batches ...
Embodiment 2
[0121] Example 2 14-O-[(5-aminopyridine)-3-thioacetyl]-mutriptyline
[0122] (a) 3-bromo-5-(tert-butoxycarbonyl)aminopyridine
[0123]
[0124] Dissolve 5-bromonicotinic acid (2g, 9.9mmol) in dry toluene (20ml), add tert-butanol (20ml), diphenylphosphoryl azide (3.2ml, 14.9mmol), triethylamine (4.1ml , 29.6mmol), under the protection of argon, reacted at 60°C for 40min, then raised the temperature to 100°C, and refluxed for 4h. Cool to room temperature, evaporate the solvent under reduced pressure, add water and ethyl acetate to the residue, shake, separate the ethyl acetate layer, dry over anhydrous sodium sulfate, filter off the desiccant, evaporate ethyl acetate, and Silica gel column chromatography gave the target compound (1.7 g, 63%).
[0125]1 H NMR (300MHz, CDCl 3 )δppm 8.33-8.32 (m, 3H), 6.77 (br, 1H), 1.53 (s, 9H) MS (EI) m / z: 272 (M) + .
[0126] (b) 14-O-[(5-aminopyridine)-3-thioacetyl]-mutriptyline
[0127]
[0128] Using 3-bromo-5-(tert-butoxycarbonyl...
Embodiment 3
[0130] Example 3 14-O-[(5-hydroxypyridine)-3-thioacetyl]-mutriptyline
[0131] (a) 3-bromo-5-(dimethyltert-butylsilyloxy)pyridine
[0132]
[0133] Dissolve 3-bromo-5-hydroxypyridine (150mg, 0.87mmol) in DMF (5ml), add TBDMSCl (170mg, 1.13mmol), imidazole (89mg, 1.31mmol), DMAP (5.2mg, 0.04mmol), Ar Under gas protection, the reaction was stirred at room temperature for 12 h. Dilute with ethyl acetate, add water, shake to separate the liquid, dry the organic phase over anhydrous sodium sulfate, filter, and evaporate to dryness to give a yellow oil (210mg, 84%).
[0134] 1 H NMR (300MHz, CDCl 3 )δ8.29(d,J=2.2Hz,1H),8.14(d,2.3Hz,1H),7.33(t,J=2.1Hz,1H)H),1.00(s,9H),0.25(s, 6H) MS (EI) m / z: 287 (M) + .
[0135] (b) 14-O-[[5-(Dimethyltert-butylsilyloxy)pyridine]-3-thioacetyl]-mutriptyline
[0136]
[0137] Using 3-bromo-5-(dimethyltert-butylsilyloxy)pyridine as raw material, the target compound can be obtained according to steps f and g in Example 1.
[0138] 1 H NMR ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Solubility | aaaaa | aaaaa |
| Solubility | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com