Electrolyte solution and electrochemical component using same
An electrolyte and electrolyte technology, applied in the field of electrolyte, can solve the problems of low conductivity, low energy density, and low capacity, and achieve the effects of high conductivity, high energy density, and high electrostatic capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
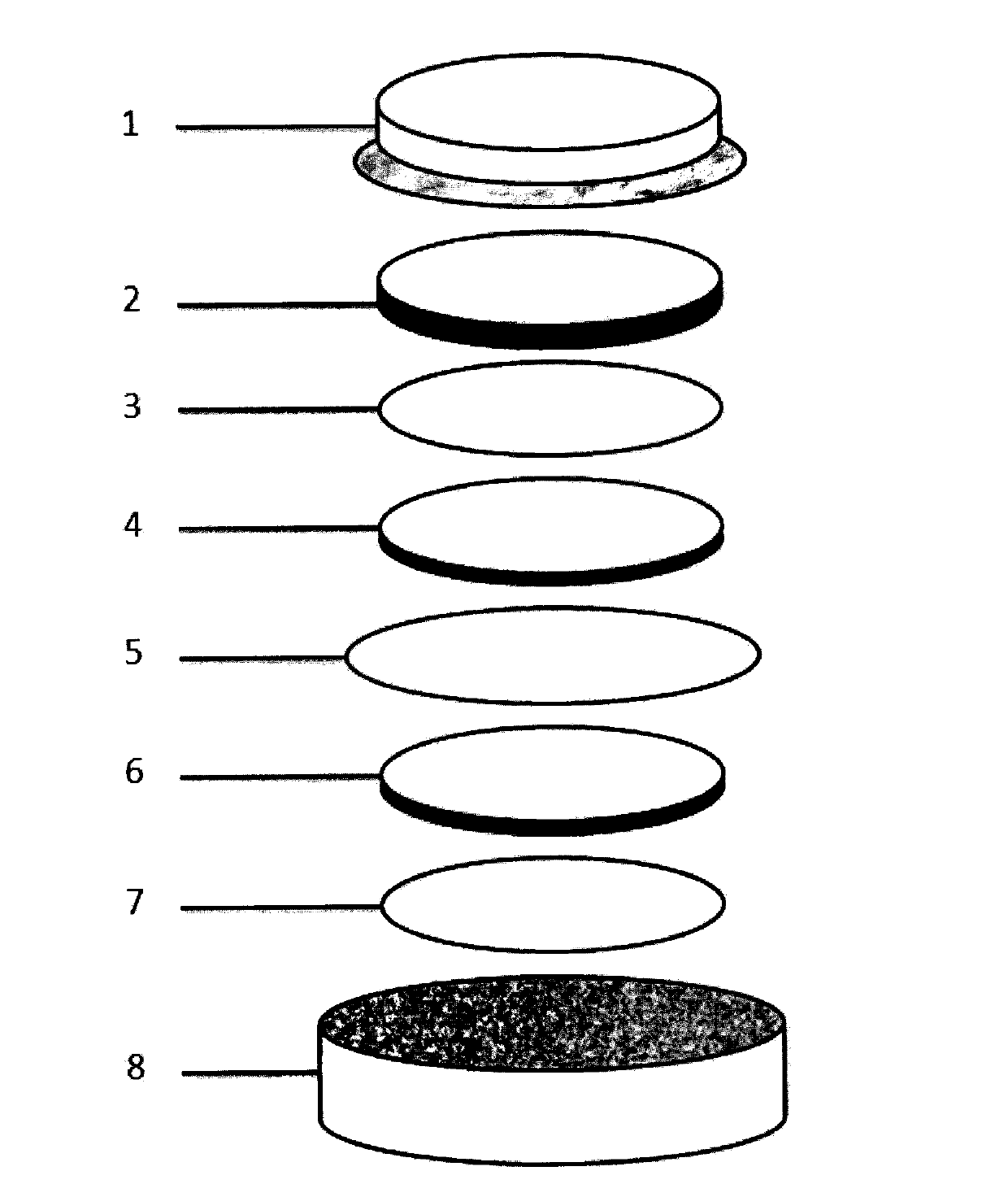
Image
Examples
Embodiment 1
[0082] (1) Electrolyte dehydration
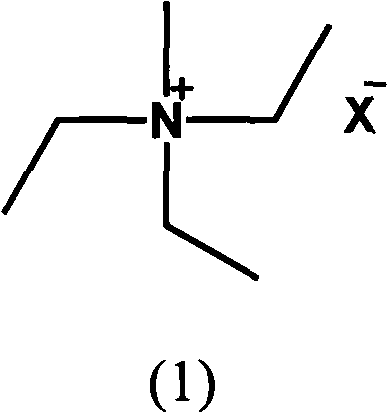
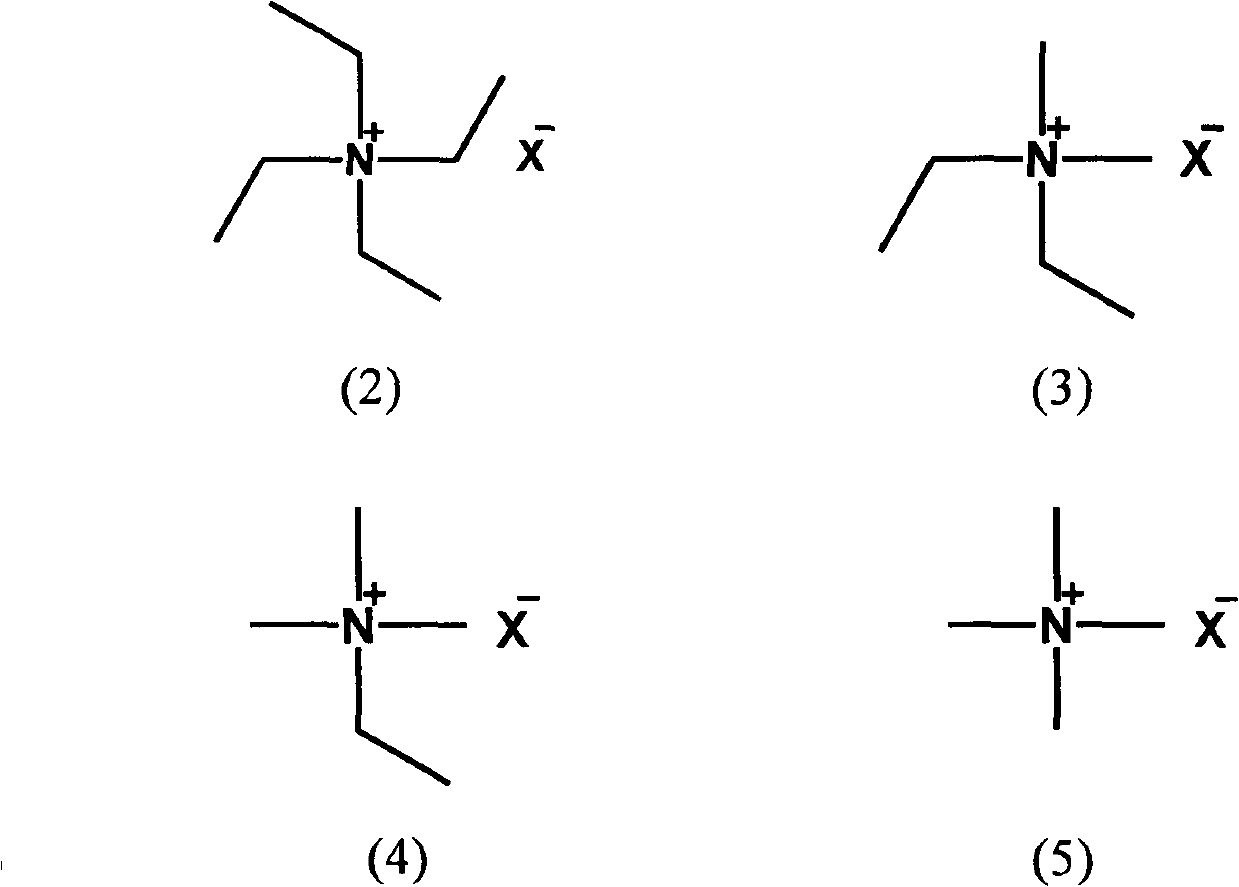
[0083] 30 parts of methyltriethylammonium tetrafluoroborate (A1), 10 parts of dimethyldiethylammonium tetrafluoroborate (B3), 5 parts of trimethylethylammonium tetrafluoroborate (B5) and 5 parts of tetrafluoroborate Tetramethylammonium fluoroborate (B7) was dried and dehydrated under reduced pressure at 130° C. for 3 hours.
[0084] (2) Electrolyte solvent dehydration
[0085] Add 5 parts of 3A type molecular sieves to 100 parts of propylene carbonate, place at 25°C for 60 hours, after drying, filter out the molecular sieves to obtain dehydrated propylene carbonate.
[0086] (3) Preparation of electrolyte
[0087] 80 parts of dehydrated propylene carbonate, 19 parts of methyltriethylammonium tetrafluoroborate (A1), 0.8 parts of dimethyldiethylammonium tetrafluoroborate (B3), trimethylethylammonium tetrafluoroborate (B5 ) and 0.1 part of tetramethylammonium tetrafluoroborate (B7) were uniformly mixed and dissolved at 25°C to obtain the el...
Embodiment 2
[0089] (1) Electrolyte dehydration
[0090] 30 parts of methyltriethylammonium tetrafluoroborate (A1), 10 parts of dimethyldiethylammonium tetrafluoroborate (B3) and 10 parts of trimethylethylammonium tetrafluoroborate (B5) at 130 ° C Dry and dehydrate under reduced pressure for 3 hours.
[0091] (2) Electrolyte solvent dehydration
[0092] Add 5 parts of 3A type molecular sieves to 100 parts of propylene carbonate and 100 parts of dimethyl carbonate respectively, place at 25°C for 60 hours, after drying, filter out the molecular sieves to obtain dehydrated propylene carbonate and dimethyl carbonate.
[0093] (3) Preparation of electrolyte
[0094] 40 parts of dehydrated propylene carbonate, 40 parts of dimethyl carbonate, 18 parts of methyltriethylammonium tetrafluoroborate (A1), 1.5 parts of dimethyldiethylammonium tetrafluoroborate (B3) and trifluoroborate 0.5 part of methylethylammonium (B5) was uniformly mixed and dissolved at 25° C. to obtain the electrolyte solution ...
Embodiment 3
[0096] (1) Electrolyte dehydration
[0097] 30 parts of methyltriethylammonium tetrafluoroborate (A1) and 10 parts of dimethyldiethylammonium tetrafluoroborate (B3) were dried and dehydrated under reduced pressure at 130° C. for 3 hours.
[0098] (2) Electrolyte solvent dehydration
[0099] Add 5 parts of 3A type molecular sieves to 100 parts of sulfolane, place at 25°C for 60 hours, after drying, filter out the molecular sieves to obtain dehydrated propylene carbonate.
[0100] (3) Preparation of electrolyte
[0101] 80 parts of dehydrated sulfolane, 19 parts of methyltriethylammonium tetrafluoroborate (A1) and 1 part of dimethyldiethylammonium tetrafluoroborate (B3) were uniformly mixed and dissolved at 25°C to obtain the electrolyte solution of the present invention ( 3). The water content of the electrolytic solution (3) was 37 ppm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com