Methods for synthesizing and purifying 1, 2, 3, 4-tetrafluorohexafluorobutane
A technology of tetrachlorohexafluorobutane and synthesis method, which is applied in chemical instruments and methods, halogenated hydrocarbon disproportionation separation/purification, organic chemistry, etc. Difficulty, separation difficulty and other problems, to achieve the effect of short process route, reduced side reactions, high economic value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
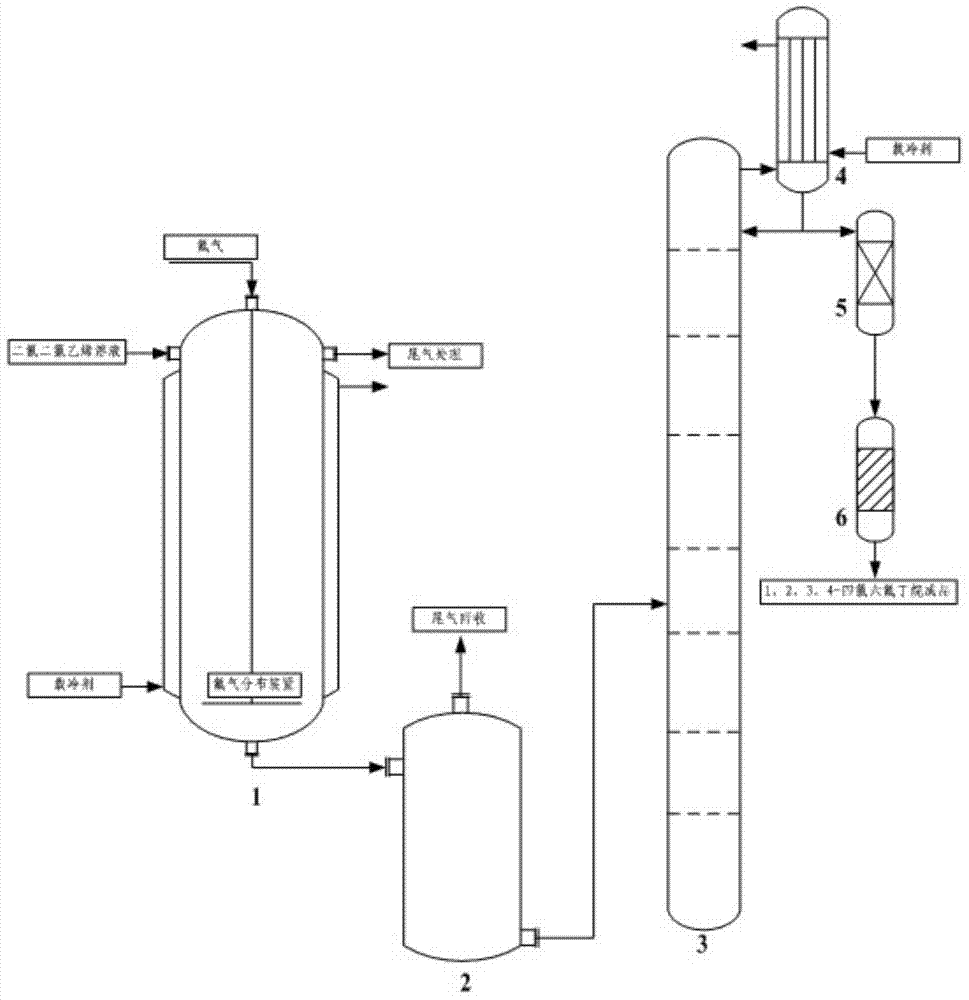
Image
Examples
Embodiment 11
[0042] Synthesis and purification of embodiment 11,2,3,4-tetrachlorohexafluorobutane
[0043] In a 316L self-made reactor with a volume of 10L, 1,2-dichlorotetrafluoroethane is used as a solvent and the content of 1,2-difluoro-1,2-dichloroethylene is 50% (volume percent) solution 5L, the fluorine gas prepared by electrolysis was treated with dehydrofluoride and potassium dehydrofluoride, pressurized to 0.15Mpa, the fluorine gas was diluted to 40% (volume percentage) with nitrogen, and introduced at a flow rate of 200L / h 1,2-difluoro-1,2-dichloroethylene in 1,2-dichlorotetrafluoroethane solution continued to react for 9 hours, during the reaction, the internal pressure of the reaction device was maintained at 0.05Mpa, and the liquid phase of the reaction system was maintained The temperature is -75±1°C. After the reaction was terminated, the liquid-phase reaction solution was taken and analyzed by gas chromatography. The analysis results are as follows: the conversion rate of...
Embodiment 21
[0045] Example 21 Synthesis and purification of 2,3,4-tetrachlorohexafluorobutane
[0046] In a 316L self-made reactor with a volume of 10L, 1,2-dichlorotetrafluoroethane is used as a solvent and the content of 1,2-difluoro-1,2-dichloroethylene is 50% (volume percentage) solution 5L, the fluorine gas prepared by electrolysis is treated with dehydrofluoride and potassium dehydrofluoride, pressurized to 0.07Mpa, the fluorine gas is diluted to 60% (volume percentage) with helium, and the flow rate is 200L / h Introduce 1,2-difluoro-1,2-dichloroethylene in 1,2-dichlorotetrafluoroethane solution and continue to react for 7 hours. During the reaction, the pressure in the reaction device is maintained at 0.02Mpa, and the liquid of the reaction system is maintained. The phase temperature is at -75±1°C. After the reaction was terminated, the liquid-phase reaction solution was taken and analyzed by gas chromatography. The analysis results were as follows: the conversion rate of 1,2-difl...
Embodiment 31
[0048] Example 31 Synthesis and purification of 2,3,4-tetrachlorohexafluorobutane
[0049] In a 316L self-made reactor with a volume of 10L, 1,2-dichlorotetrafluoroethane is used as a solvent and the content of 1,2-difluoro-1,2-dichloroethylene is 50% (volume percent) solution 5L, the fluorine gas prepared by electrolysis was treated with dehydrofluorination and potassium dehydrofluoride, pressurized to 0.15Mpa, the fluorine gas was diluted to 40% (volume percentage) with argon gas, and the flow rate was 200L / h Introduce 1,2-difluoro-1,2-dichloroethylene in 1,2-dichlorotetrafluoroethane solution and continue to react for 9 hours. During the reaction, the pressure in the reaction device is maintained at 0.05Mpa, and the liquid of the reaction system is maintained. The phase temperature is at -45±1°C. After the reaction was terminated, the liquid-phase reaction solution was taken and analyzed by gas chromatography. The analysis results are as follows: the conversion rate of 1,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com