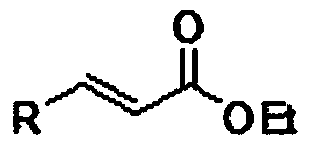
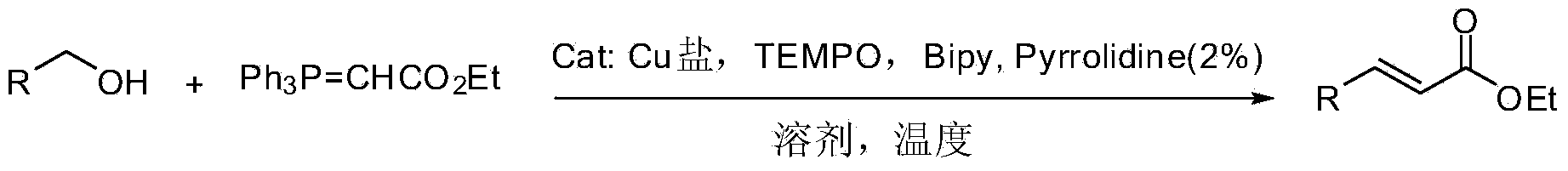
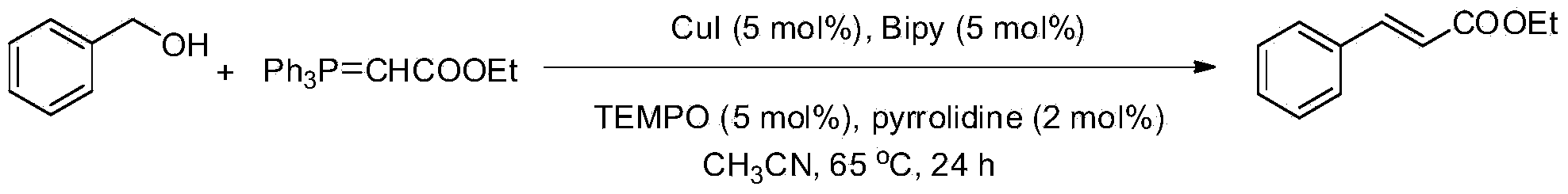Alpha, beta-unsaturated carboxylic ester compound and preparation method thereof
An ester compound, unsaturated technology, applied in the field of unsaturated carboxylic acid ester compounds, can solve the problems of reduced practicality and promotion potential, difficult preparation of oxidants, high toxicity of oxidation systems, etc., and achieves good research and industrial application prospects , Reduce synthesis cost, easy product separation and purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Utilize ethyl (triphenylphosphine) acetate to react with benzyl alcohol to prepare ethyl cinnamate,
[0027]
[0028] 0.1031mL, 1mmol of benzyl alcohol, 0.5225g, 1.5mmol of ethyl (triphenylphosphine) acetate, 0.01g, 5mol% of CuI, 0.008g, 5mol% of Bipy, 0.011g, 5mol% of TEMPO , 0.0016mL, 5mol% tetrahydropyrrole were sequentially added to a 100mL reaction tube, 2mL of acetonitrile was added as a solvent, an air balloon was added to the reaction tube, and the reaction was carried out at 65°C for 24h. The product was separated and purified by column chromatography, and the separation yield was 98%. 1HNMR (300MHz, CDCl3): δ7.68(d, J=16.2Hz, 1H), 7.52-7.49(m, 2H), 7.37-7.35(m, 3H), 6.43(d, J=15.9Hz, 1H) ,4.26(q,J=7.2Hz,2H),1.33(t,J=7.2Hz,3H).13CNMR(125.4MHz,CDCl3):δ166.7,144.4,134.4,130.0,128.7,127.9,118.2,60.3,14.2 .MS(EI):m / z(%)176(20),148(19),147(17),132(10),131(100),104(19),103(64),102(15 ), 77(44), 51(20).
Embodiment 2
[0030] Ethyl (triphenylphosphine) acetate reacts with p-methylbenzyl alcohol to prepare ethyl p-methyl cinnamate:
[0031]
[0032] 0.1220g, 1mmol of p-methyl benzyl alcohol, 0.5225g, 1.5mmol of ethyl (triphenylphosphine) acetate, 0.01g, 5mol% of CuI, 0.008g, 5mol% of Bipy, 0.011g, Add 5mol% TEMPO, 0.0016mL, and 5mol% tetrahydropyrrole to a 100mL reaction tube in turn, add 2mL acetonitrile as a solvent, add an air balloon to the reaction tube, and react at 65°C for 24h. The product was separated and purified by column chromatography, and the separation yield was 66%. 1HNMR (500MHz, CDCl3): δ7.66(d, J=16.0Hz, 1H), 7.40(d, J=8.0Hz, 2H), 7.16(d, J=8.0Hz, 2H), 6.38(d, J =16.0Hz,1H),4.25(q,J=7.0Hz,2H),2.35(s,3H),1.32(t,J=7.0Hz,3H).13CNMR(125.4MHz,CDCl3):δ167.0,144.4, 140.4, 131.7, 129.5, 127.9, 117.1, 60.2, 21.3, 14.2. MS (EI): m / z (%) 190 (41), 162 (16), 146 (11), 145 (100), 118 (26 ), 117(41), 116(14), 115(43), 91(20).
Embodiment 3
[0034] Preparation of ethyl o-methoxycinnamate by reaction of ethyl (triphenylphosphine) acetate with o-methoxybenzyl alcohol
[0035]
[0036]With 0.1328mL, 1mmol o-methoxybenzyl alcohol, 0.5225g, 1.5mmol ethyl (triphenylphosphine) acetate, 0.01g, 5mol% CuI, 0.008g, 5mol% Bipy, 0.011g, Add 5mol% TEMPO, 0.0016mL, and 5mol% tetrahydropyrrole into a 100mL reaction tube in turn, add 2mL acetonitrile as a solvent, add an air balloon to the reaction tube, and react at 65°C for 24h. The product was separated and purified by column chromatography, and the separation yield was 98%. 1HNMR (500MHz, CDCl3): δ7.99(d, J=16.5Hz, 1H), 7.51-7.49(m, 1H), 7.35-7.32(m, 1H), 6.96-6.89(m, 2H), 6.52( d,J=16.0Hz,1H),4.26(q,J=7.0Hz,2H),3.87(s,3H)1.33(t,J=7.0Hz,3H).13CNMR(125.4MHz,CDCl3):δ167. 4,158.3,139.9,131.3,128.8,120.6,118.8,111.1,60.2,55.4,14.3.MS(EI):m / z(%)206(41),176(11),175(92),162(11) ,161(100),147(88),146(18),132(15),131(26),119(24),118(37),105(39),103(23),91(28) ,90(16),89(22),79(1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com