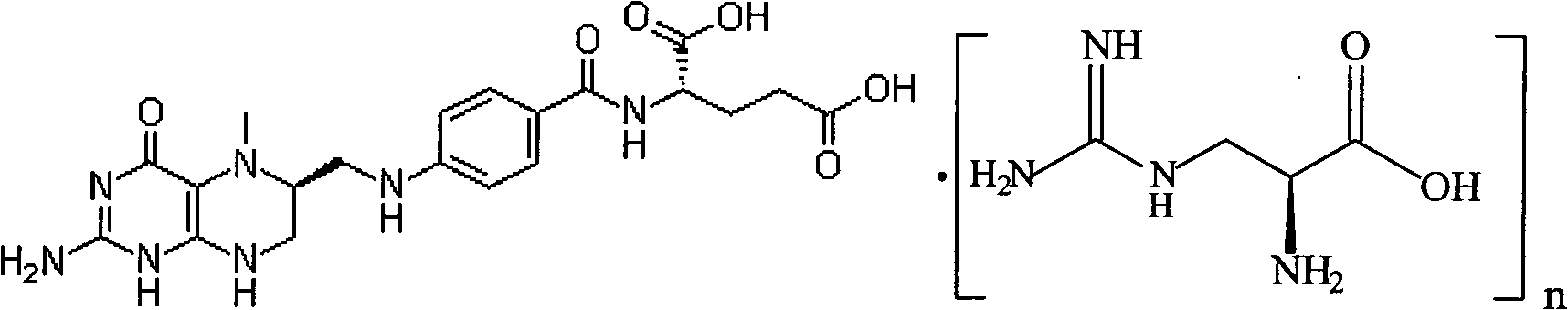
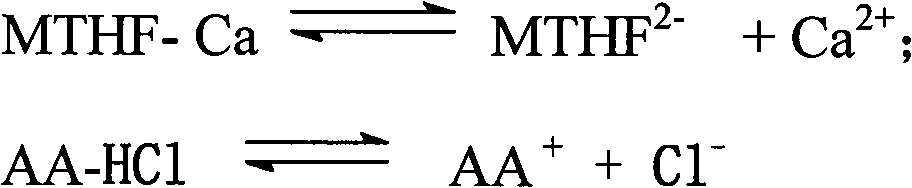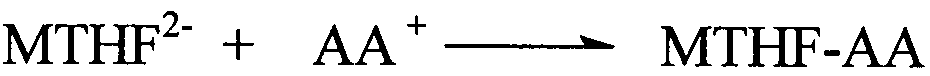Preparation method of L-5-methyl tetrahydrofolate amino acid salt
A technology of methyltetrahydrofolate and calcium methyltetrahydrofolate is applied in the field of preparation of organic medicine L-5-methyltetrahydrofolate amino acid salt, and can solve the problem of high cost, separation and purification of L-5-MTHF difficulty, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Under nitrogen flow, in a 1000ml three-necked flask, suspend 49.7g (0.1mol) L-calcium tetrahydrofolate in 200ml ethanol, and take another 21.1g (0.1mol) L-arginine hydrochloride dissolved in 200ml water acid, while stirring, slowly add to the ethanol suspension of L-calcium tetrahydrofolate, keep the temperature at 0-20°C; adjust the pH to 6-6.8 with dilute hydrochloric acid; fully stir the above mixture at room temperature for 8 hours , add 200ml of acetone or 300ml of absolute ethanol dropwise, and stir for another 6 hours; let it stand for 24 hours; filter, wash with cold ethanol / water, and dry under vacuum at 20-40°C. Obtained 45.9 g of L-arginine salt crystalline powder of L-5-methyltetrahydrofolate, yield 72.5%, chemical purity 98.1%, [α] 20 D =+40.1° (C=1.5, water) optical purity ee≥99.0%. HNMR(D2O): 7.68(2H); 6.72(2H); 4.36(1H); 3.74(1H) 3.696(1H); 3.46(1H); 3.10~3.01(3H); 3.206(2H); 2.54(3H) ; 2.35(2H); 2.099+2.19(2H); 1.840(2H); 1.668(2H).
Embodiment 2
[0031] Under nitrogen flow, in a 1000ml three-necked flask, suspend 49.7g (0.1mol) of L-calcium tetrahydrofolate in 200ml of ethanol, and take another 17.4g (0.1mol) of arginine dissolved in 200ml of water, while While stirring, slowly add to the ethanol suspension of L-calcium tetrahydrofolate, keep the temperature at 0-20°C, adjust the pH to 6-6.8 with dilute hydrochloric acid; stir the above mixture at room temperature for 8 hours, dropwise 200ml of acetone or 300ml of absolute ethanol, and stir for another 6 hours; let stand for 24 hours; filter, wash with cold ethanol / water, and vacuum dry at 20-40°C. Obtained 48.2 g of L-5-methyltetrahydrofolate arginine salt crystalline powder, yield 71.8%, chemical purity 98.2%, [α] 20 D =+43.1 ° (C=1.5, water), optical purity ee≥99.0%.HNMR (D2O): 7.68 (2H); 6.72 (2H); 4.36 (1H); 3.74 (1H) 3.696 (1H); 3.46 ( 1H); 3.10~3.01(3H); 3.206(2H); 2.54(3H); 2.35(2H); 2.099+2.19(2H); 1.840(2H); 1.668(2H).
Embodiment 3
[0033] Under nitrogen flow, in a 1000ml three-neck flask, suspend 49.7g (0.1mol) L-calcium tetrahydrofolate in 200ml ethanol, and take another 20.9g (0.1mol) L-histamine hydrochloride dissolved in 200ml water acid, while stirring, slowly add to the ethanol suspension of L-calcium tetrahydrofolate, keep the temperature at 0-20°C; adjust the pH to 6.0-6.8 with dilute hydrochloric acid; fully stir the above mixture at room temperature for 8 hours , add 200ml of acetone or 300ml of absolute ethanol dropwise, and stir for another 6 hours; let it stand for 24 hours; filter, wash with cold ethanol / water, and dry under vacuum at 20-40°C. Obtained 45.6 g of crystalline powder of L-5-methyltetrahydrofolate L-histidine salt, yield 72.2%, chemical purity 98.3%, [α] 20 D=+40.1° (C=1.5, water) optical purity ee≥99.0%. HNMR(D2O): 7.90(1H); 7.68(2H); 7.09(1H); 6.72(2H); 4.36(1H); 3.89(1H); 3.696(1H); 3.46(1H); 3.16(1H); 3.24(1H); 3.10~3.01(3H); 2.54(3H); 2.35(2H); 2.099+2.19(2H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com