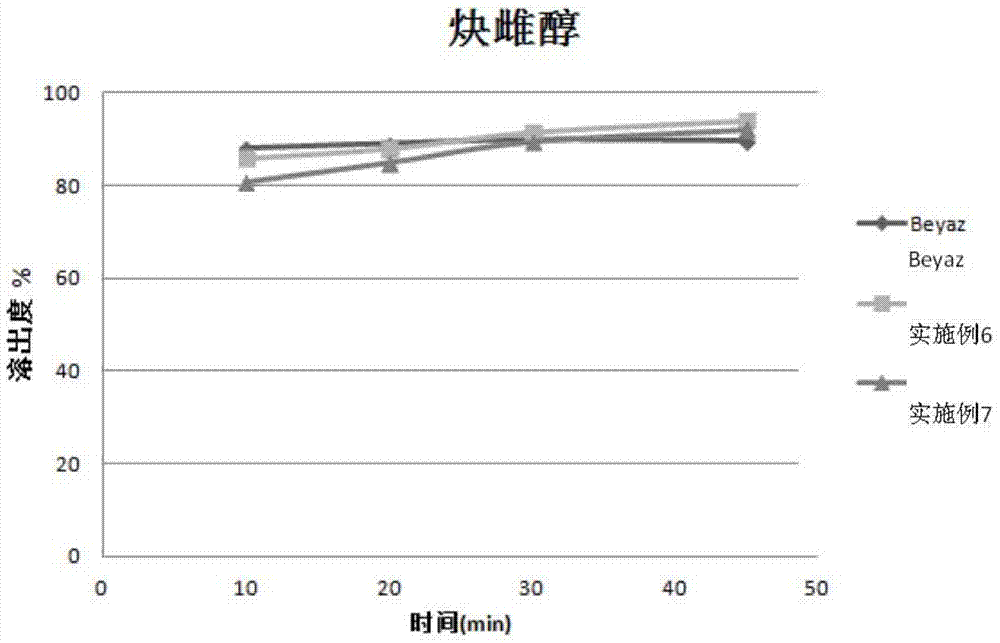
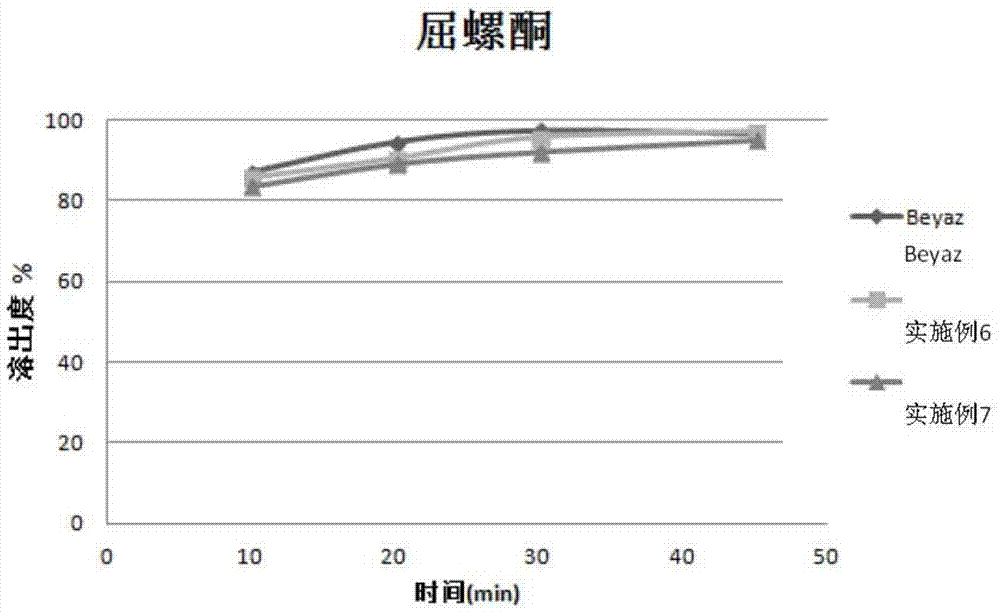
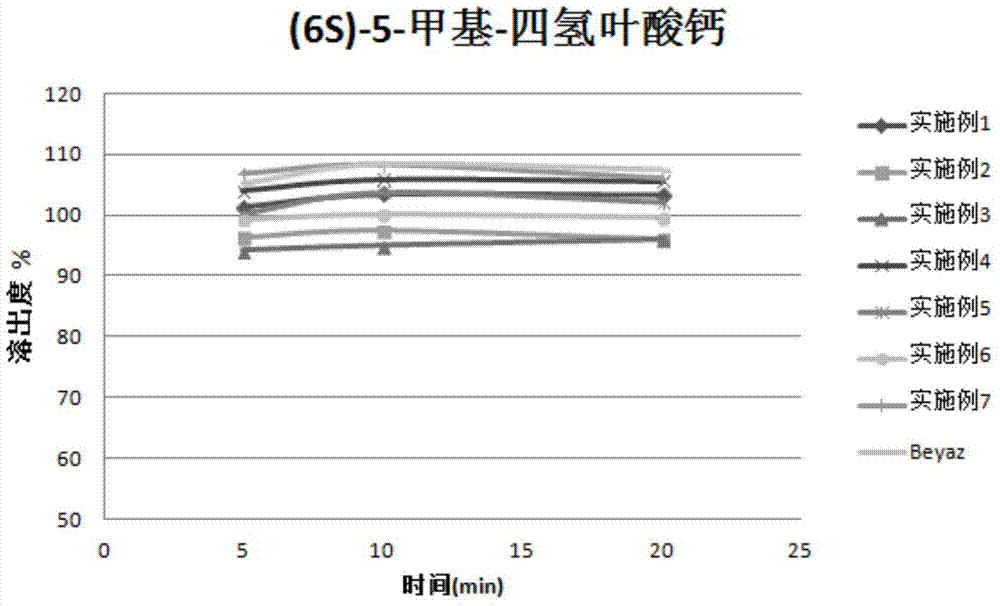
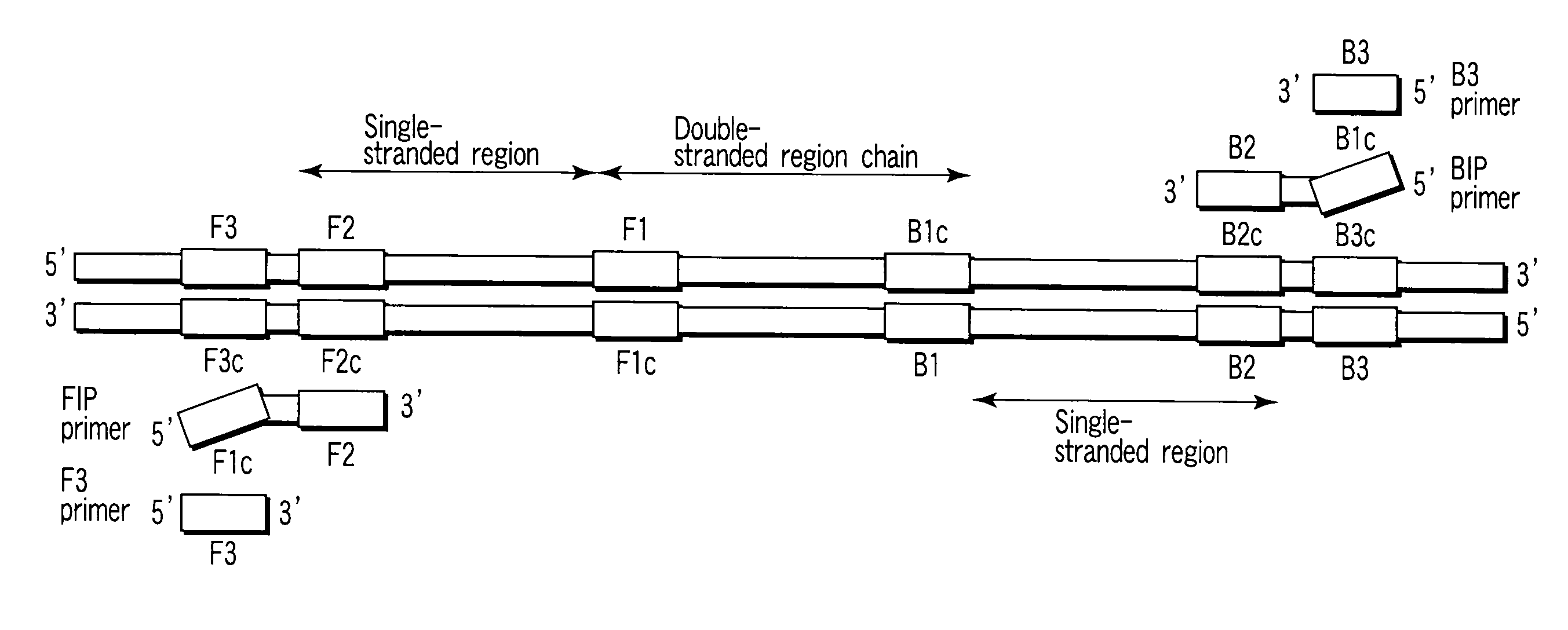
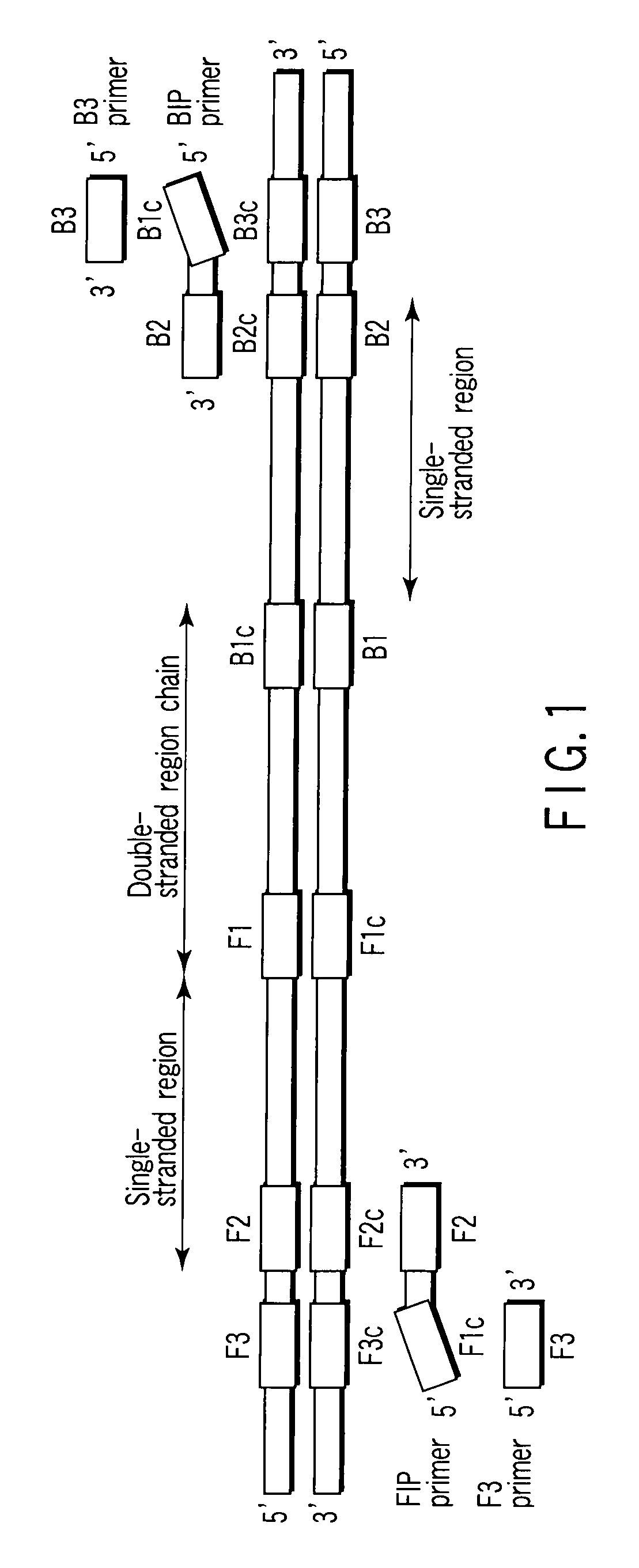
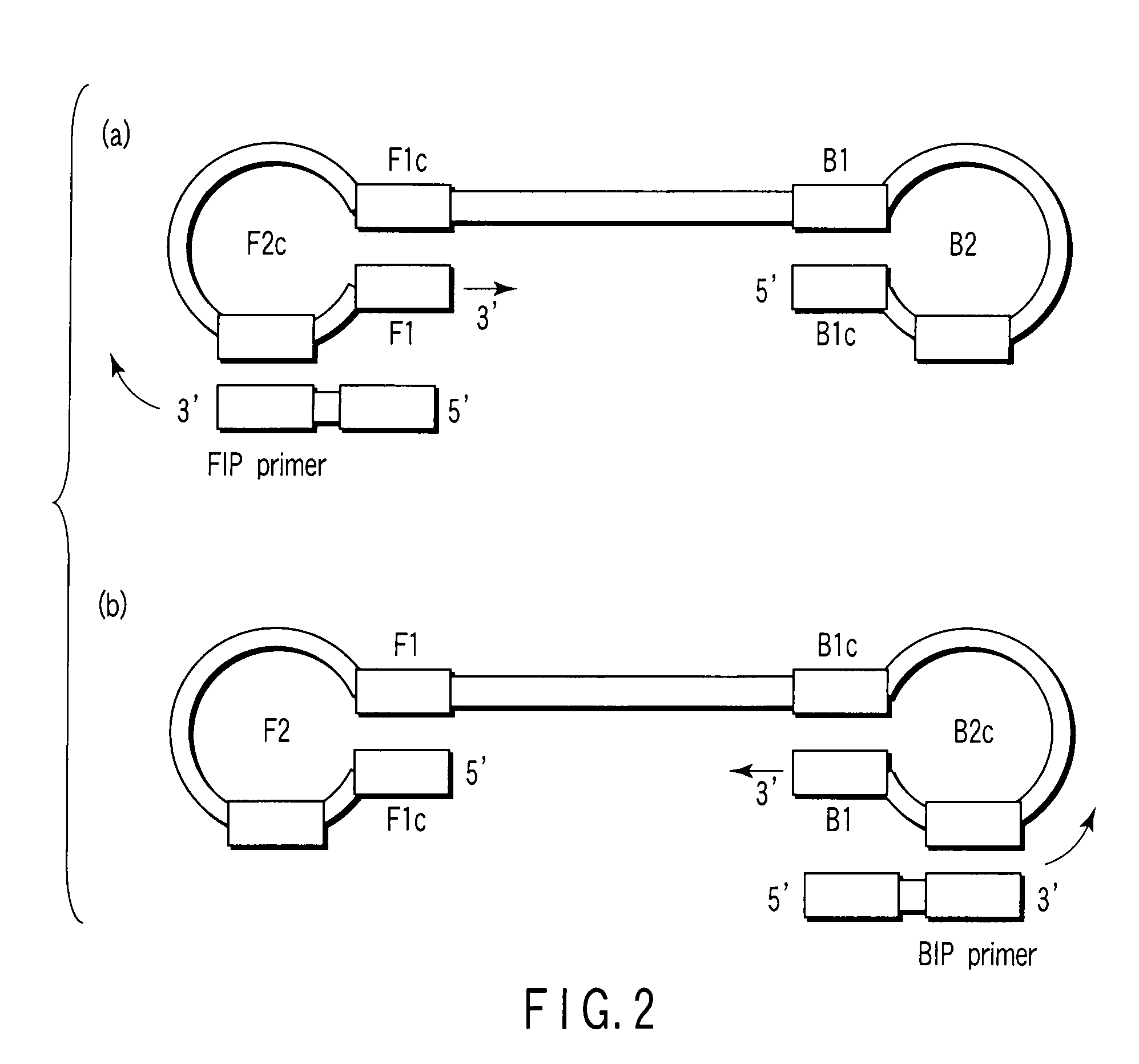
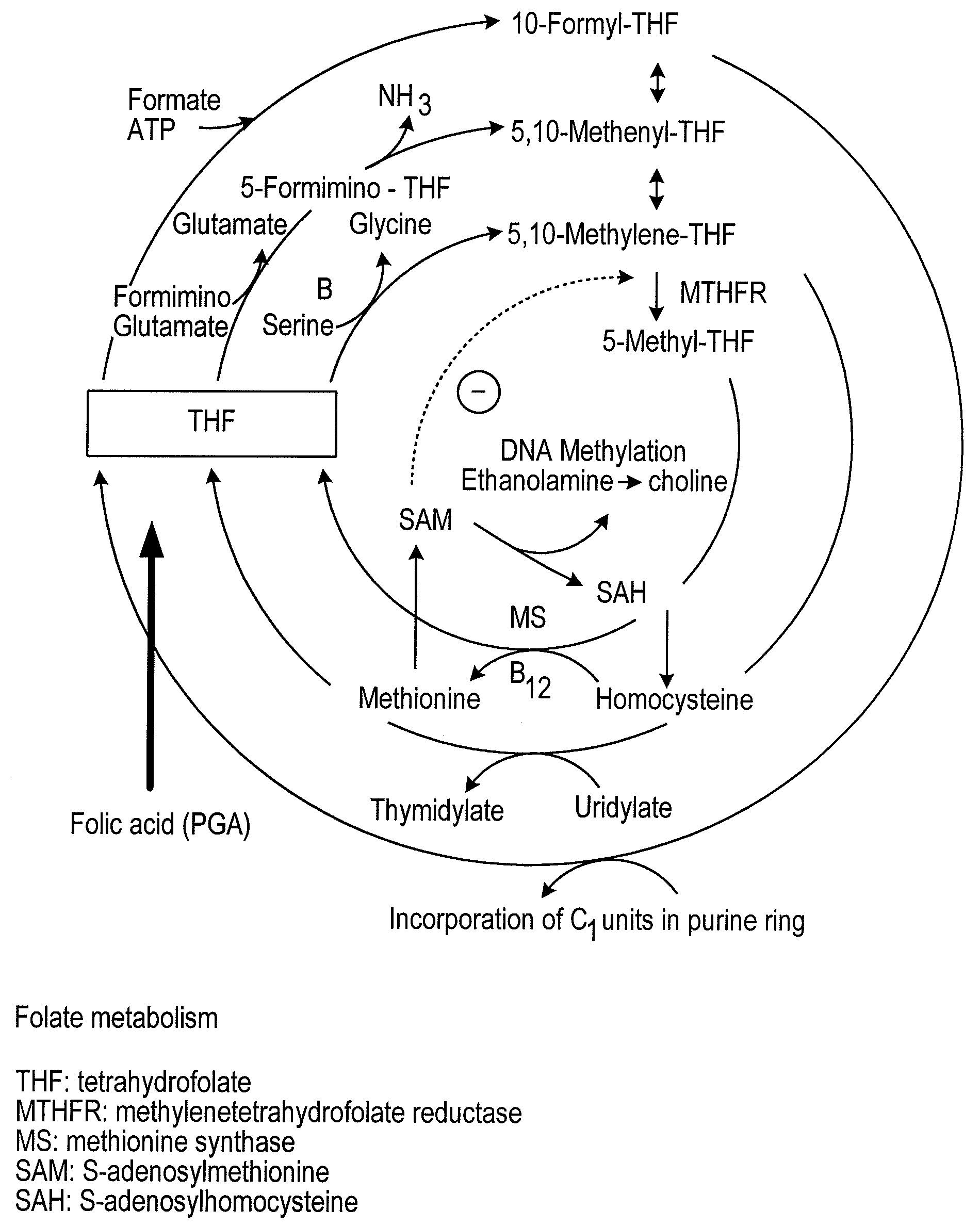
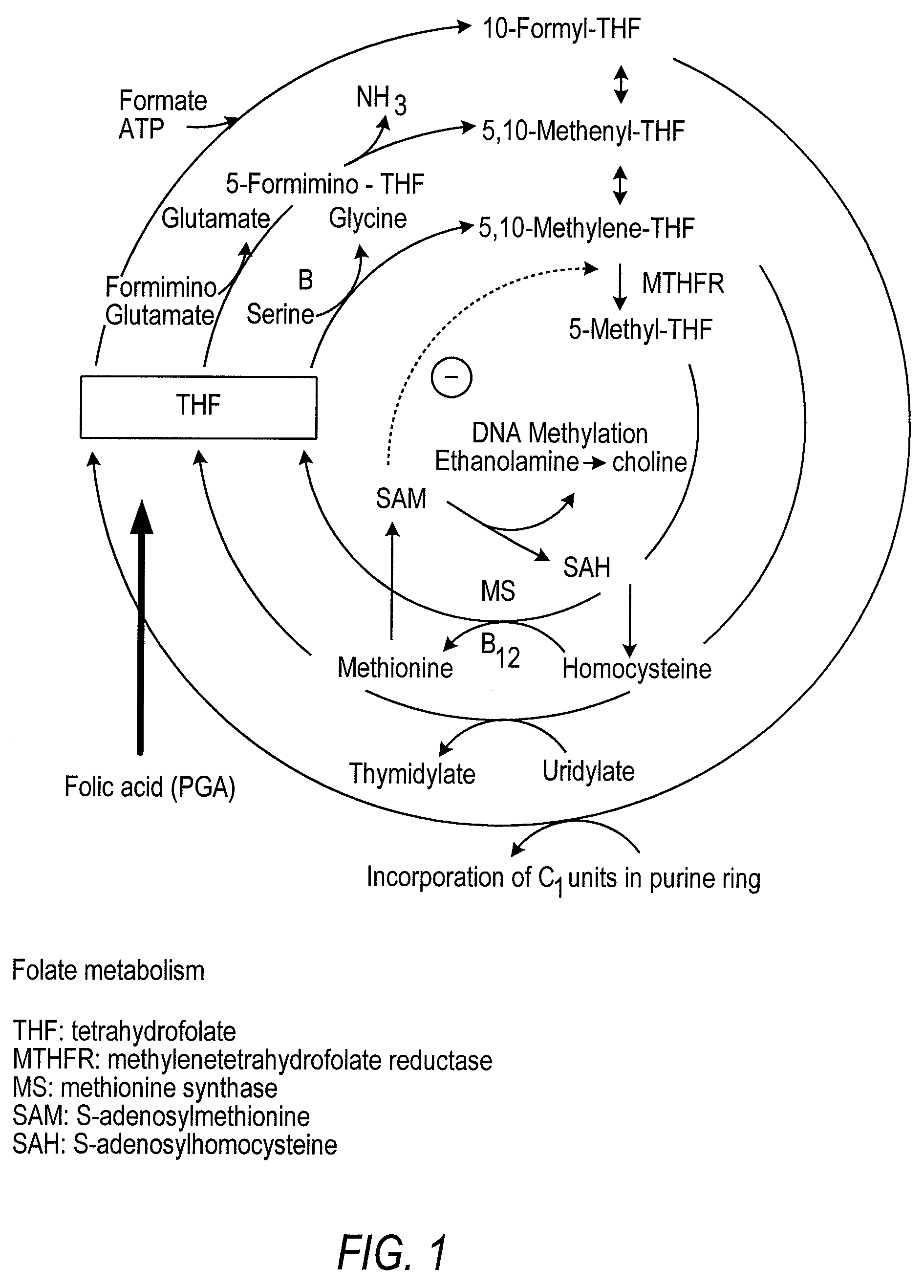
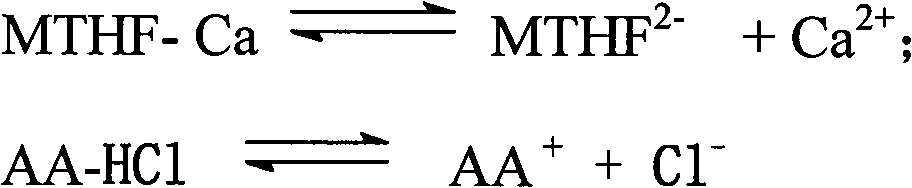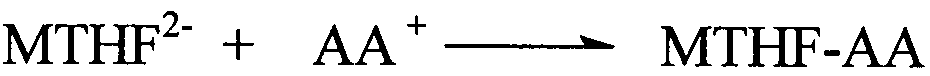Patents
Literature
87 results about "Tetrahydrofolates" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
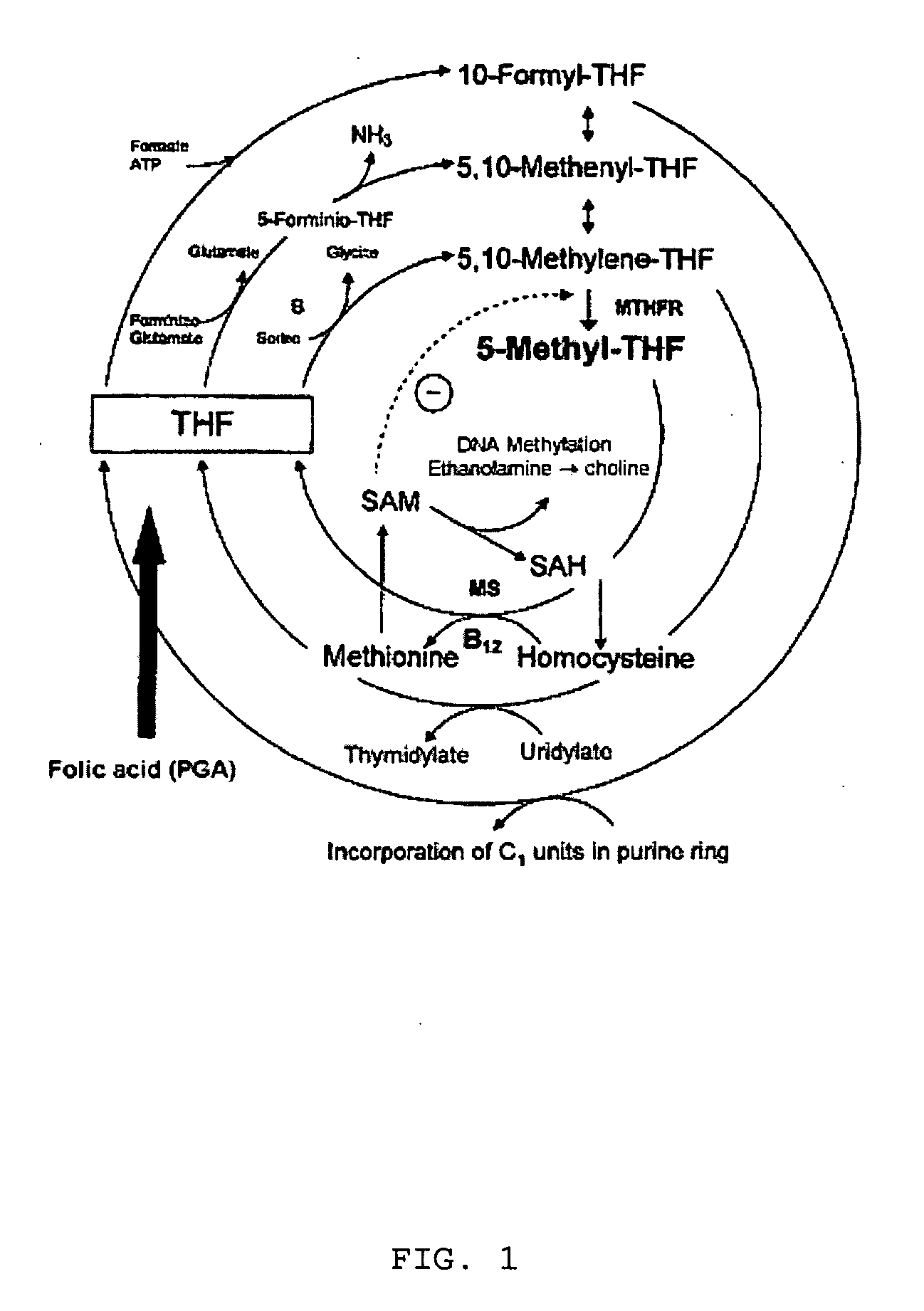
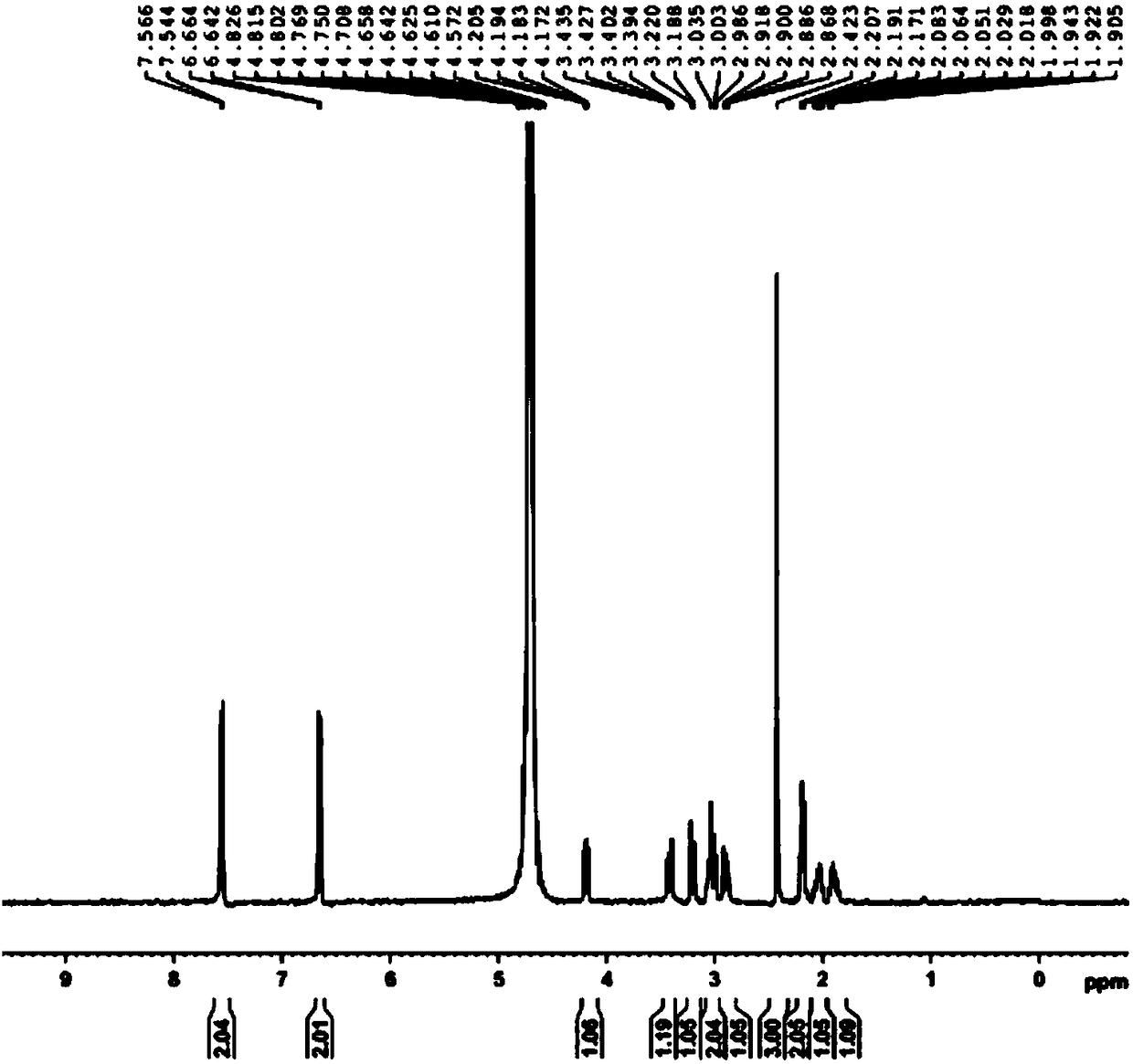
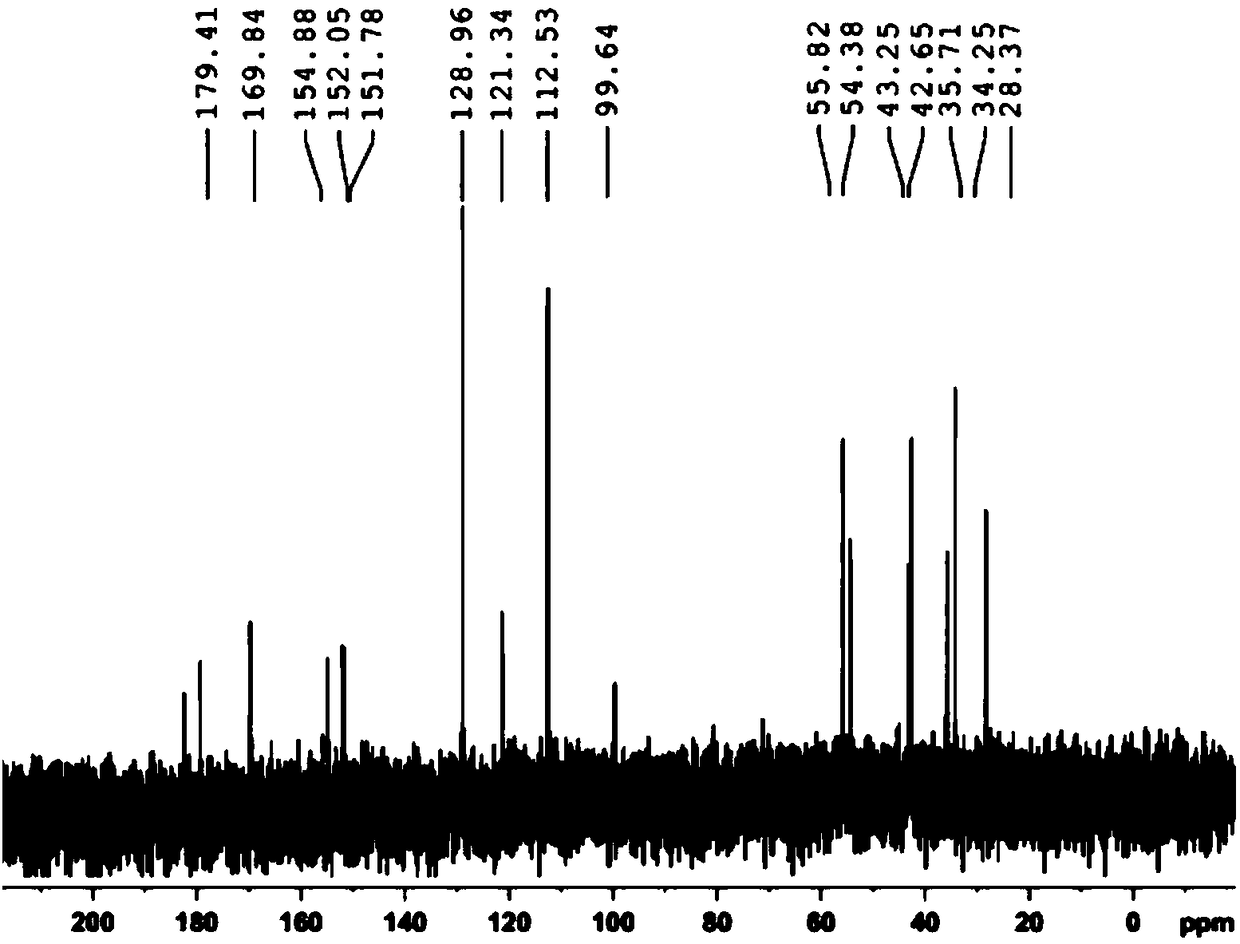
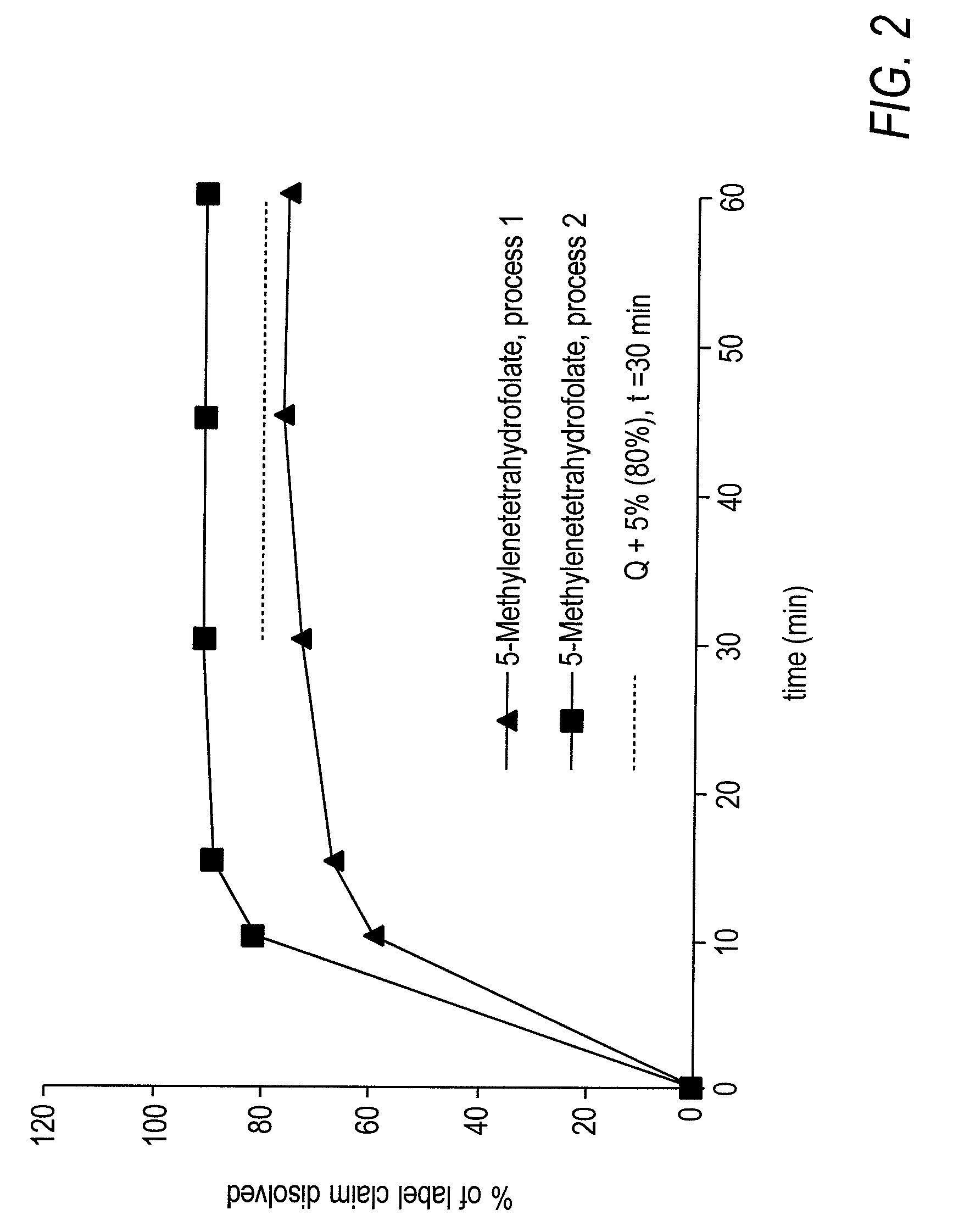
Compounds based on 5,6,7,8-tetrahydrofolate.
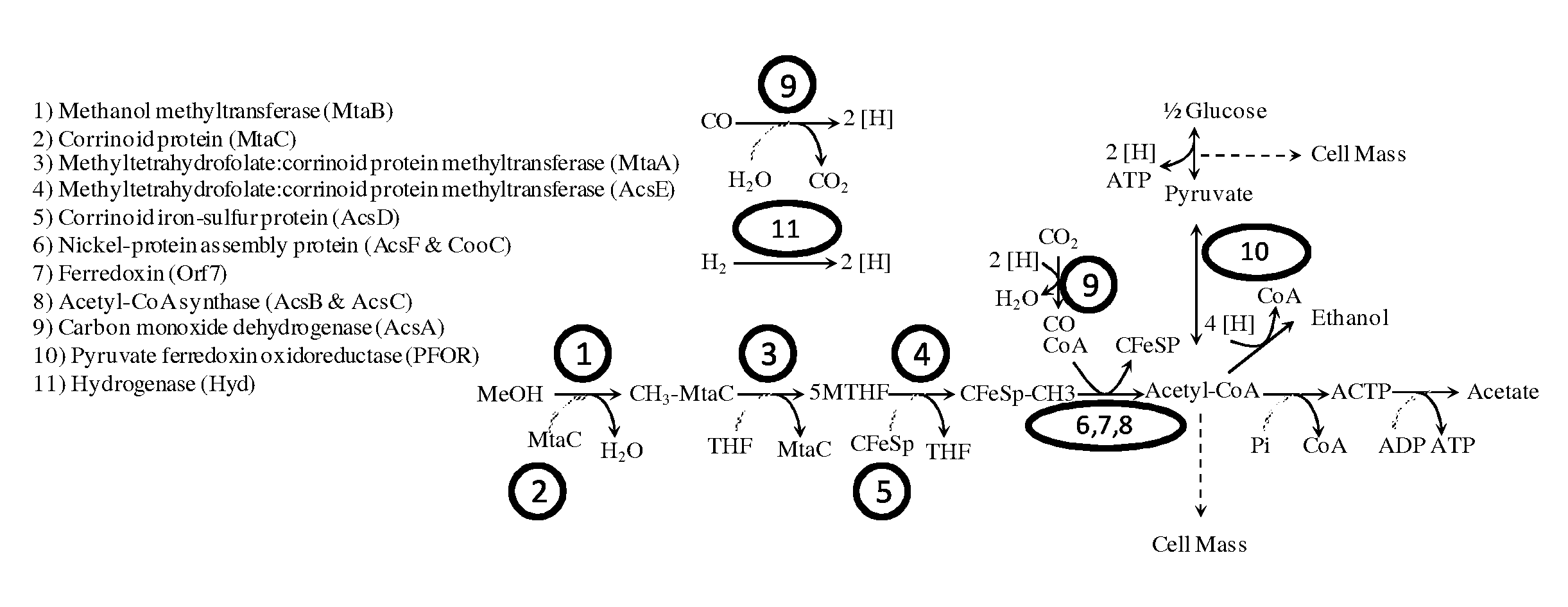
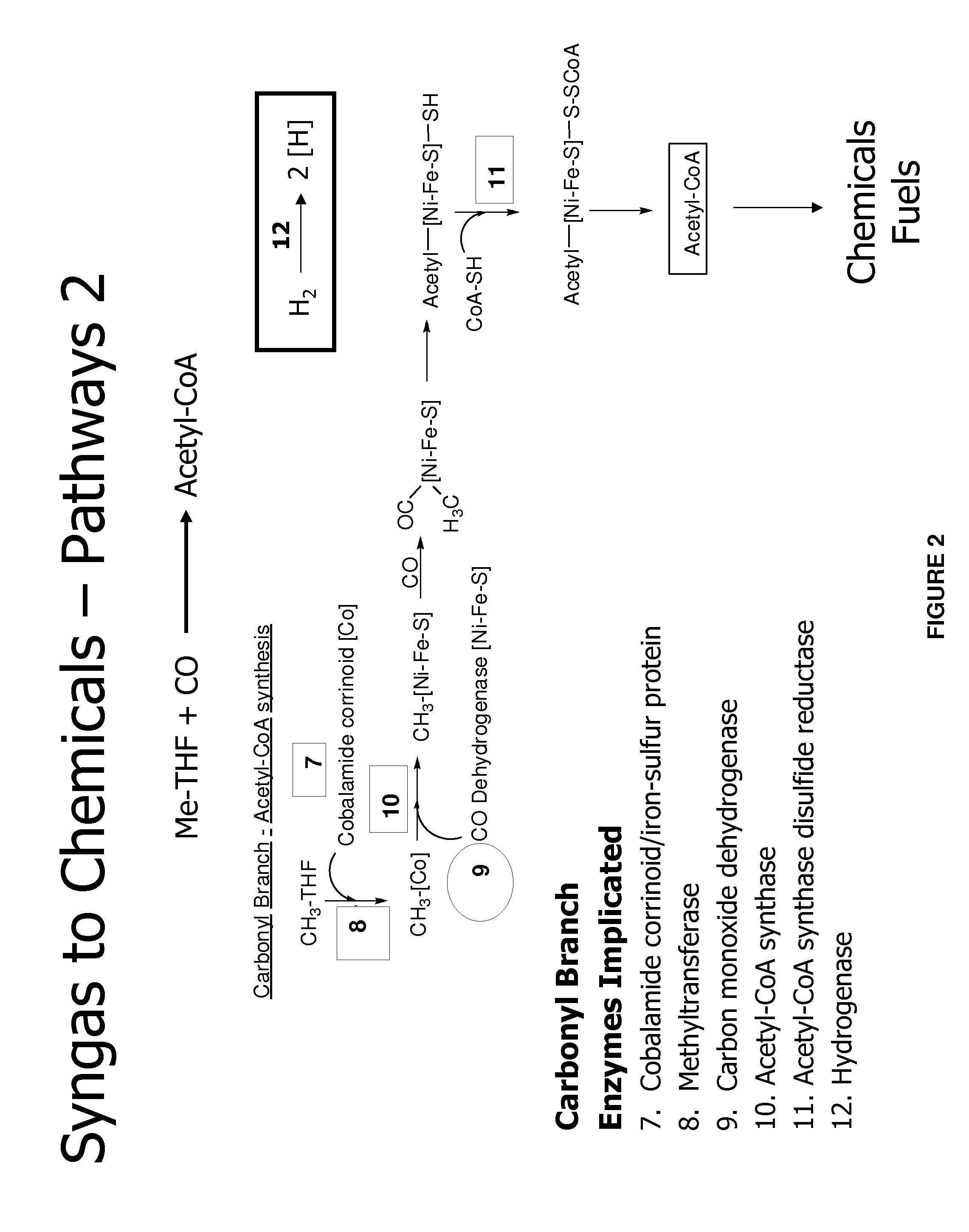
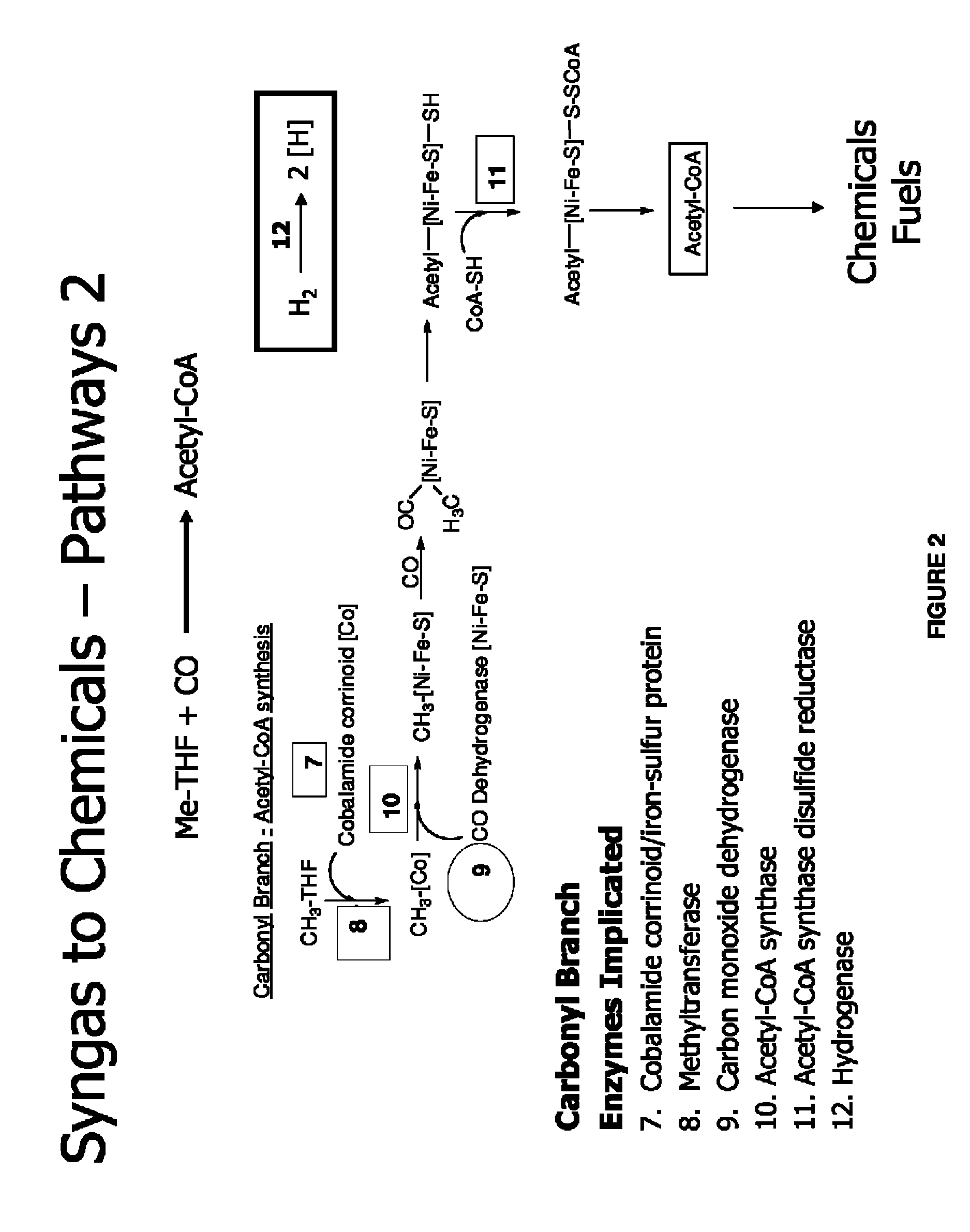
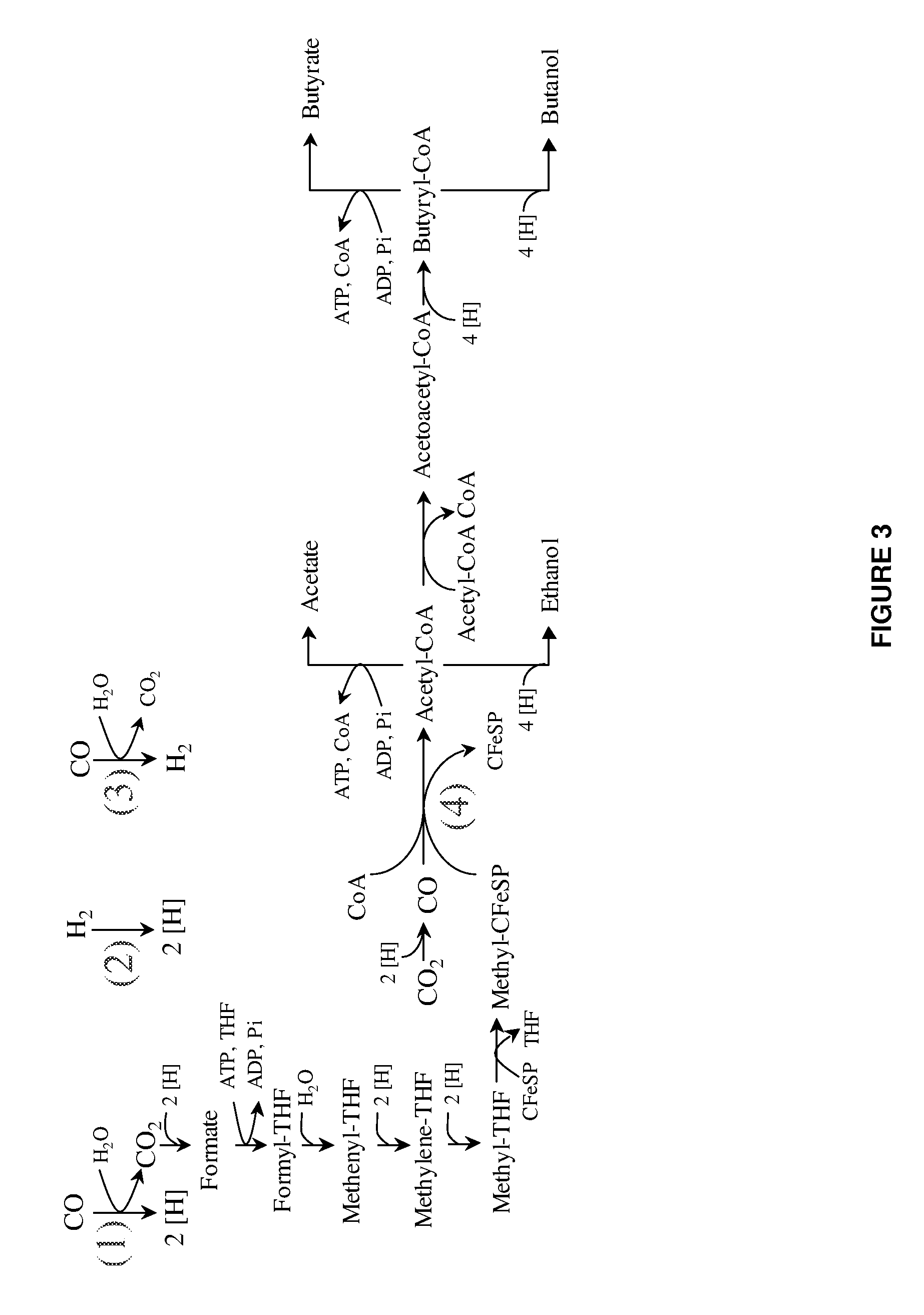
Methods and organisms for utilizing synthesis gas or other gaseous carbon sources and methanol
The invention provides a non-naturally occurring microbial organism having an acetyl-CoA pathway and the capability of utilizing syngas or syngas and methanol. In one embodiment, the invention provides a non-naturally occurring microorganism, comprising one or more exogenous proteins conferring to the microorganism a pathway to convert CO, CO2 and / or H2 to acetyl-coenzyme A (acetyl-CoA), methyl tetrahydrofolate (methyl-THF) or other desired products, wherein the microorganism lacks the ability to convert CO or CO2 and H2 to acetyl-CoA or methyl-THF in the absence of the one or more exogenous proteins. For example, the microbial organism can contain at least one exogenous nucleic acid encoding an enzyme or protein in an acetyl-CoA pathway. The microbial organism is capable of utilizing synthesis gases comprising CO, CO2 and / or H2, alone or in combination with methanol, to produce acetyl-CoA. The invention additionally provides a method for producing acetyl-CoA, for example, by culturing an acetyl-CoA producing microbial organism, where the microbial organism expresses at least one exogenous nucleic acid encoding an acetyl-CoA pathway enzyme or protein in a sufficient amount to produce acetyl-CoA, under conditions and for a sufficient period of time to produce acetyl-CoA.
Owner:GENOMATICA INC
Pharmaceutical compositions comprising one or more steroids one or more tetrahydrofolate components and vitamin b12
InactiveUS20050164977A1Avoid problemsBiocideCarbohydrate active ingredientsOral medicationPhysiology
The present invention is concerned with a kit for use in a hormonal contraceptive method or hormone replacement therapy in mammalian females, said kit comprising at least 10 oral dosage units containing at least 1 μg of one or more steroids selected from the group consisting of estrogens and progestogens; at least 0.1 mg of one or more tetrahydrofolate components selected from the group consisting of (6S)-tetrahydrofolic acid, 5-methyl-(6S)-tetrahydrofolic acid, 5-formyl-(6S)-tetrahydrofolic acid, 10-formyl-(6R)-tetrahydrofolic acid, 5,10-methylene-(6R)-tetrahydrofolic acid, 5,10-methenyl-(6R)-tetrahydrofolic acid, 5-formimino-(6S)-tetrahydrofolic acid, pharmaceutically acceptable salts of these tetrahydrofolic acids and glutamyl derivatives of these tetrahydrofolic acids; and at least 0.1 mg vitamin B 12. Other aspects of the present invention relate to a hormonal contraceptive method and a method of hormone replacement therapy comprising the at least once daily oral administration of one or more steroid containing dosage units to a mammalian female, wherein the dosage units additionally contain at least 0.1 mg of one or more of the aforementioned tetrahydrofolate components and at least 0.1 mg vitamin B 12.
Owner:PANTARHEI BIOSCI
Compositions and methods for the regulation of homocysteine levels within the body
InactiveUS20050171034A1Reduce harmful metabolic waste productLower Level RequirementsBiocideCarbohydrate active ingredientsMethylcobalaminBetaine
Described herein is a method for reducing levels of the harmful metabolic waste product of S-adenosylmethionine (SAMe), homocysteine, and provide vitamin and other nutritional co-factors that reduce the production of homocysteine and either re-methylate homocysteine back to S-adenosylmethionine, or facilitate its conversion downstream to cystathione. The method of the invention may be achieved by administering 5-methyl tetrahydrofolate, methylcobalamin, and one or more compounds selected from the group consisting of betaine, pyridoxal-5-phosphate, N-acetyl-cysteine, and other cofactors.
Owner:FAST BALANCE
Methods and organisms for utilizing synthesis gas or other gaseous carbon sources and methanol
The invention provides a non-naturally occurring microbial organism having an acetyl-CoA pathway and the capability of utilizing syngas or syngas and methanol. In one embodiment, the invention provides a non-naturally occurring microorganism, comprising one or more exogenous proteins conferring to the microorganism a pathway to convert CO, CO2 and / or H2 to acetyl-coenzyme A (acetyl-CoA), methyl tetrahydrofolate (methyl-THF) or other desired products, wherein the microorganism lacks the ability to convert CO or CO2 and H2 to acetyl-CoA or methyl-THF in the absence of the one or more exogenous proteins. For example, the microbial organism can contain at least one exogenous nucleic acid encoding an enzyme or protein in an acetyl-CoA pathway. The microbial organism is capable of utilizing synthesis gases comprising CO, CO2 and / or H2, alone or in combination with methanol, to produce acetyl-CoA. The invention additionally provides a method for producing acetyl-CoA, for example, by culturing an acetyl-CoA producing microbial organism, where the microbial organism expresses at least one exogenous nucleic acid encoding an acetyl-CoA pathway enzyme or protein in a sufficient amount to produce acetyl-CoA, under conditions and for a sufficient period of time to produce acetyl-CoA.
Owner:GENOMATICA INC
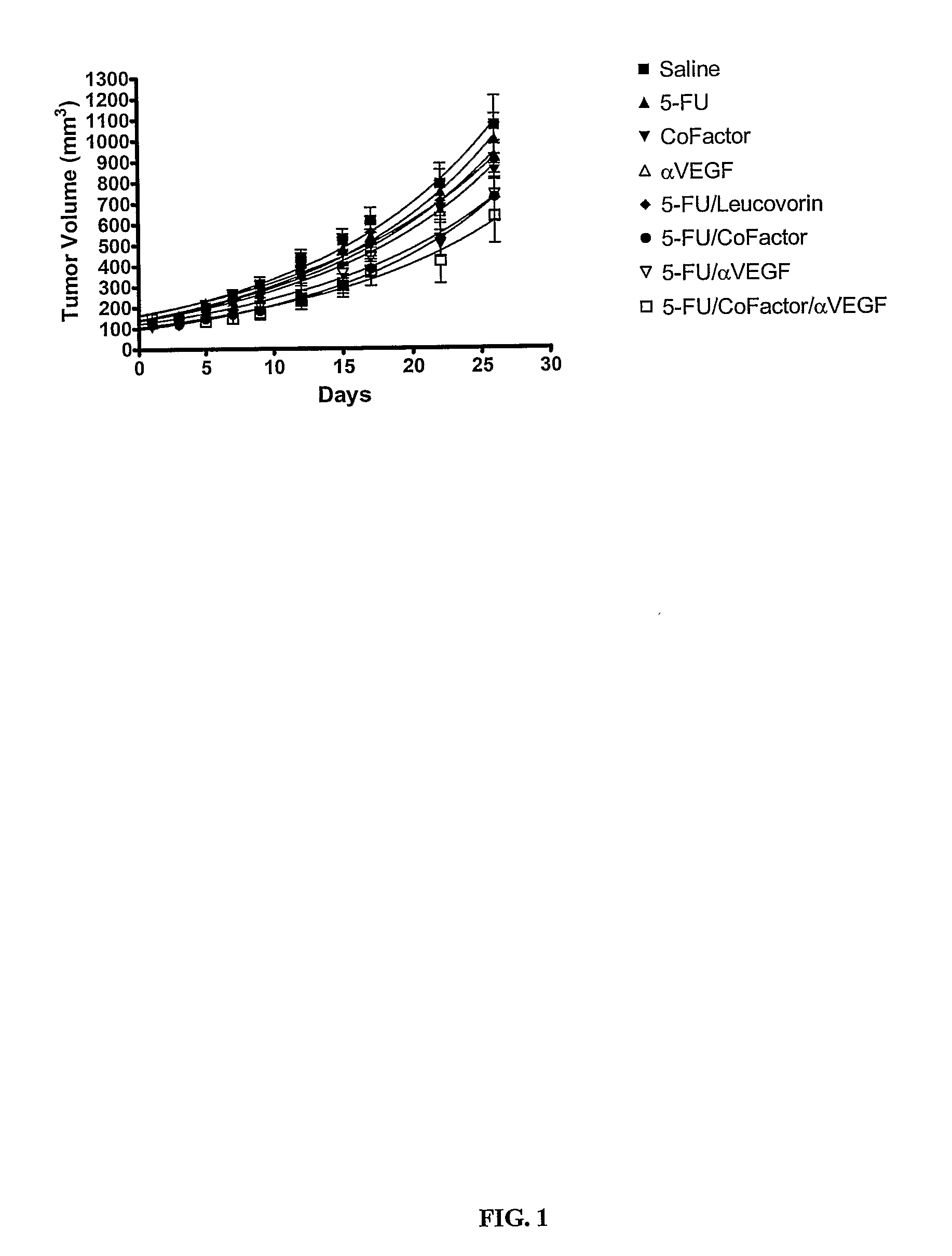
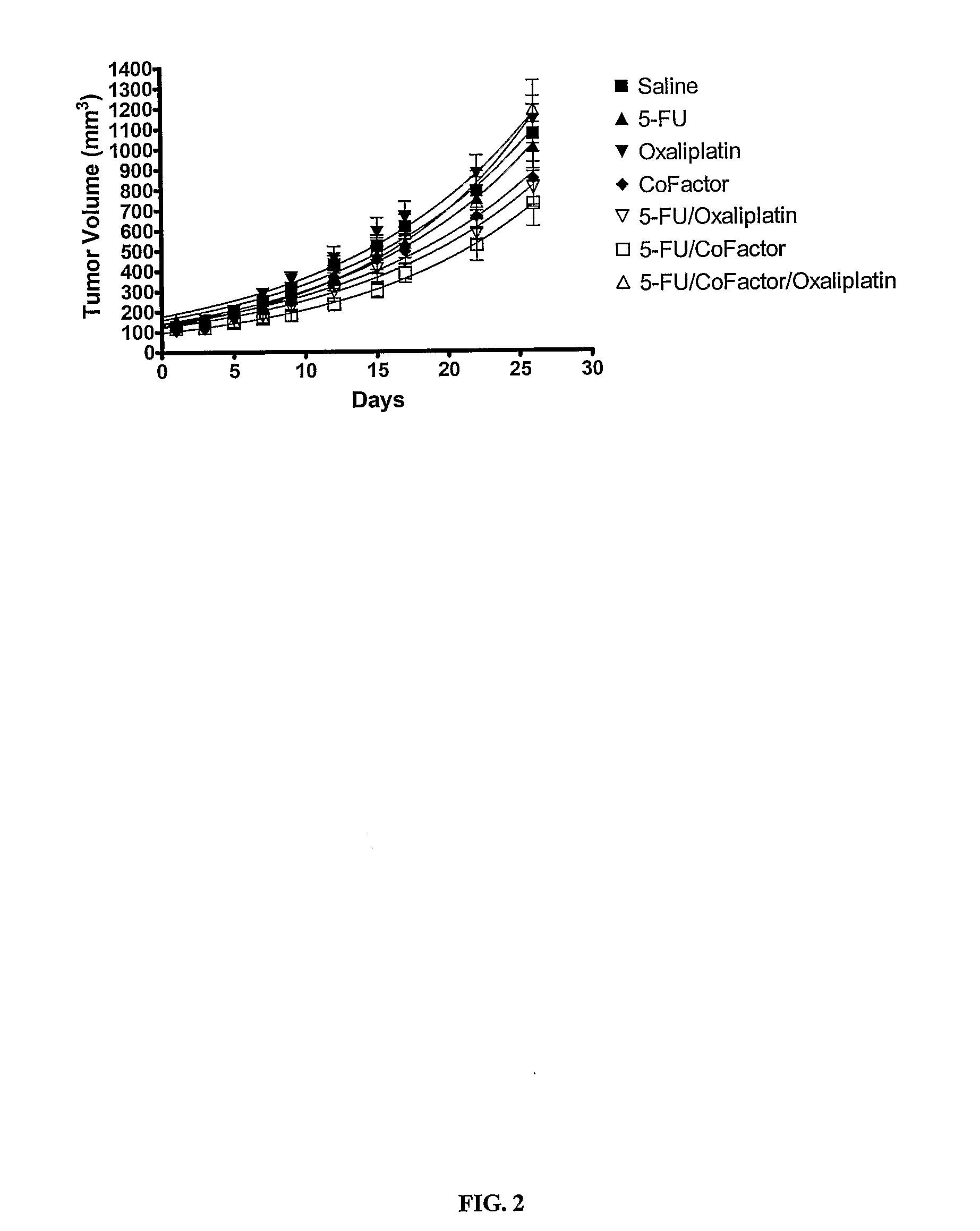
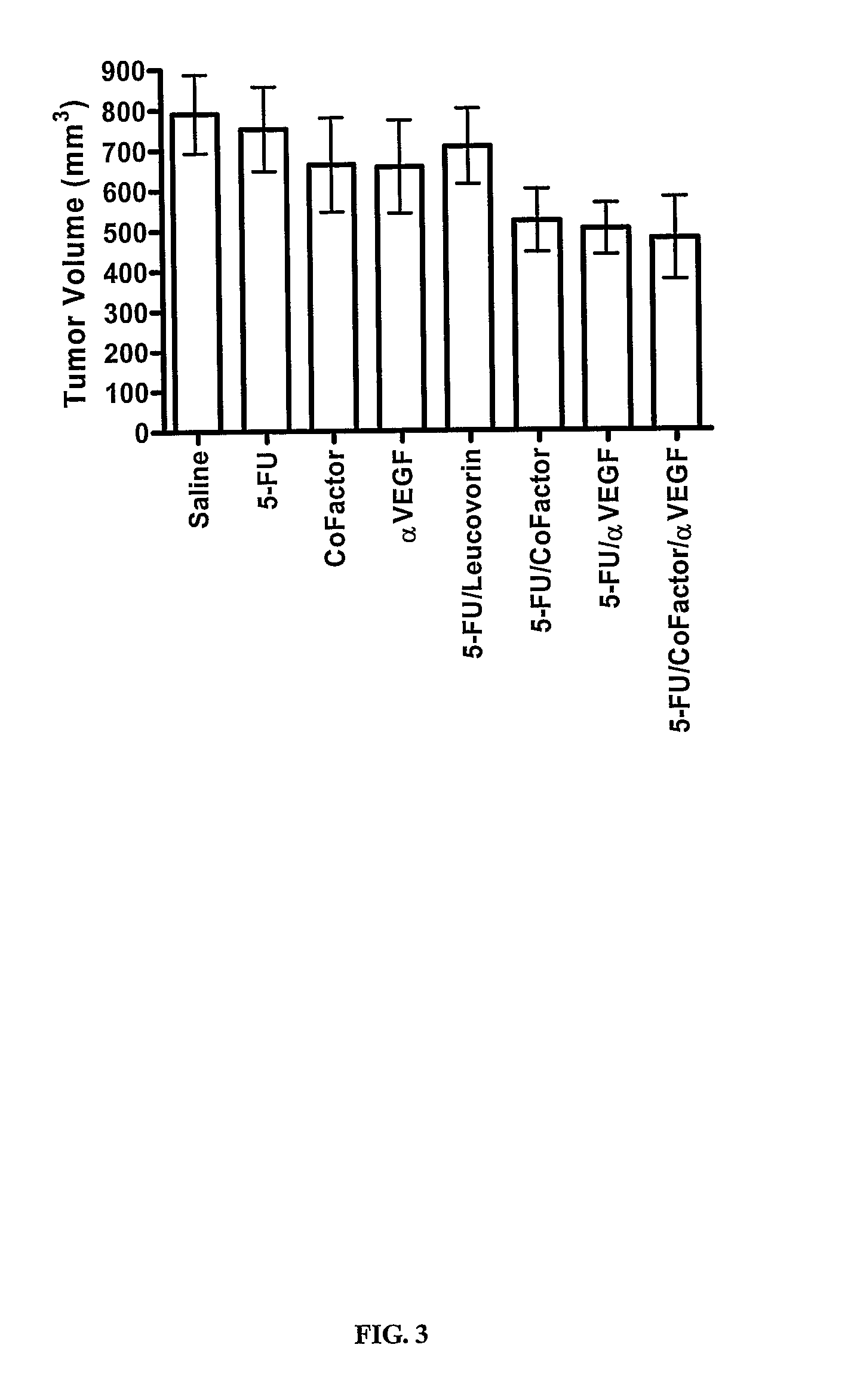
Use of 5,10-Methylene Tetrahydrofolate for the Treatment of Cancer
InactiveUS20070280944A1Improve anti-cancer effectGood curative effectBiocidePhosphorous compound active ingredientsMedicineTreatment modality
The present invention provides novel uses and compositions for 5,10-methylene tetrahydrofolate (“5,10-CH2-THFA”) in the treatment of cancer. The present invention is based on the surprising result that 5,10-CH2-THFA, while increasing the efficacy of 5-fluoruracil (5-FU) in reducing the rate of tumor growth and increasing survivorship, also reduces the toxicity to the patient of 5-FU. The present invention provides methods and compositions for treating cancer patients that include 5-FU, 5,10-CH2-THFA, and one or more additional anticancer drugs. Such methods and compositions can provide increased efficacy and reduced toxicity when compared with current treatment modalities.
Owner:ADVENTRX PHARMA INC
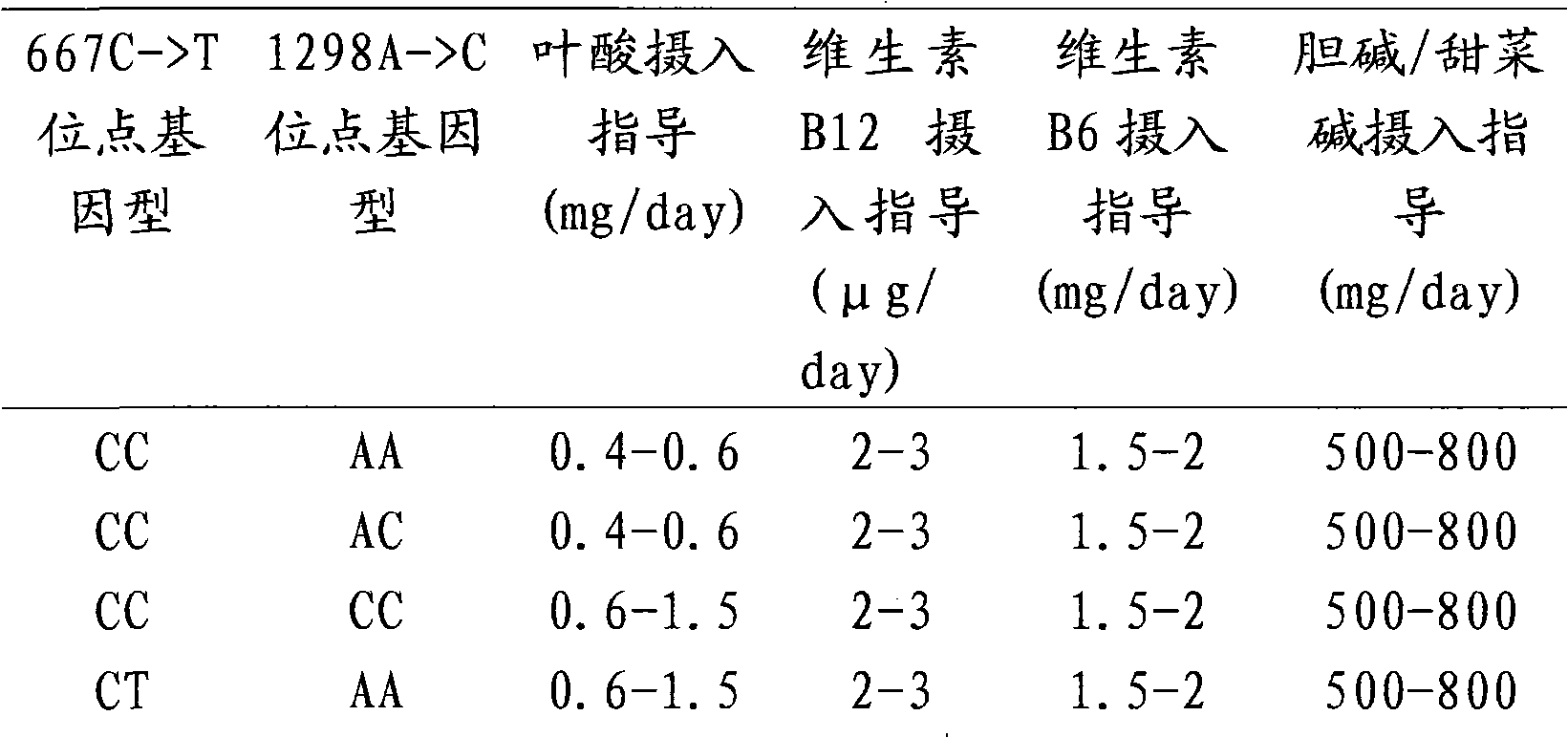
Kit for quantitative guidance of folic acid supplement dosage before and during pregnancy
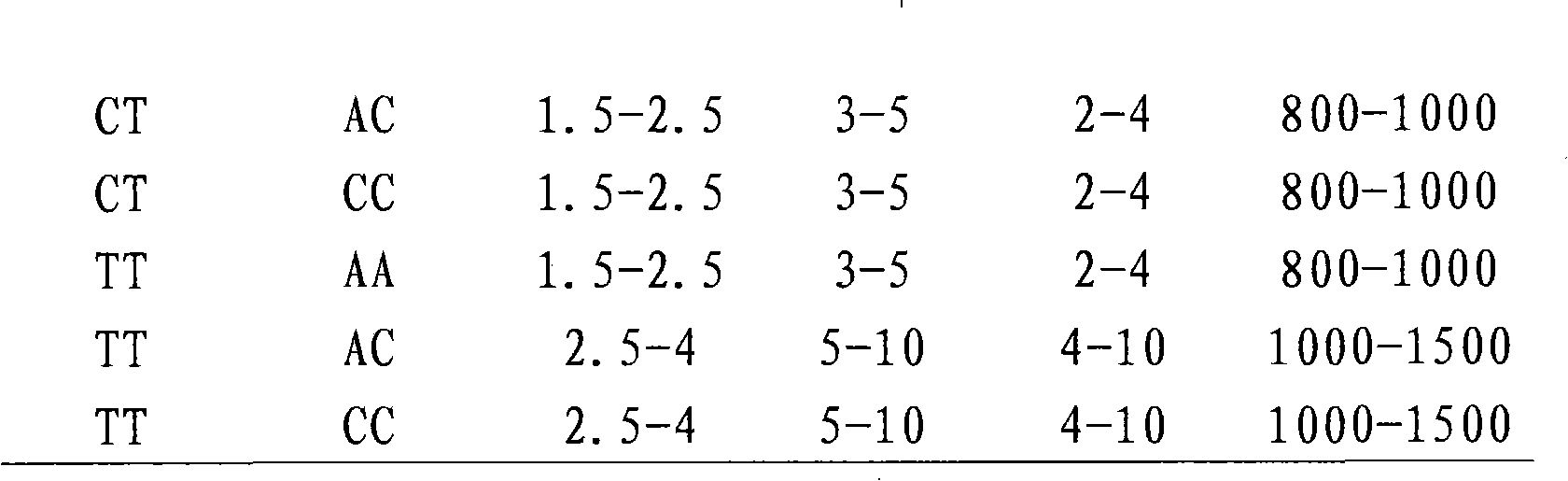
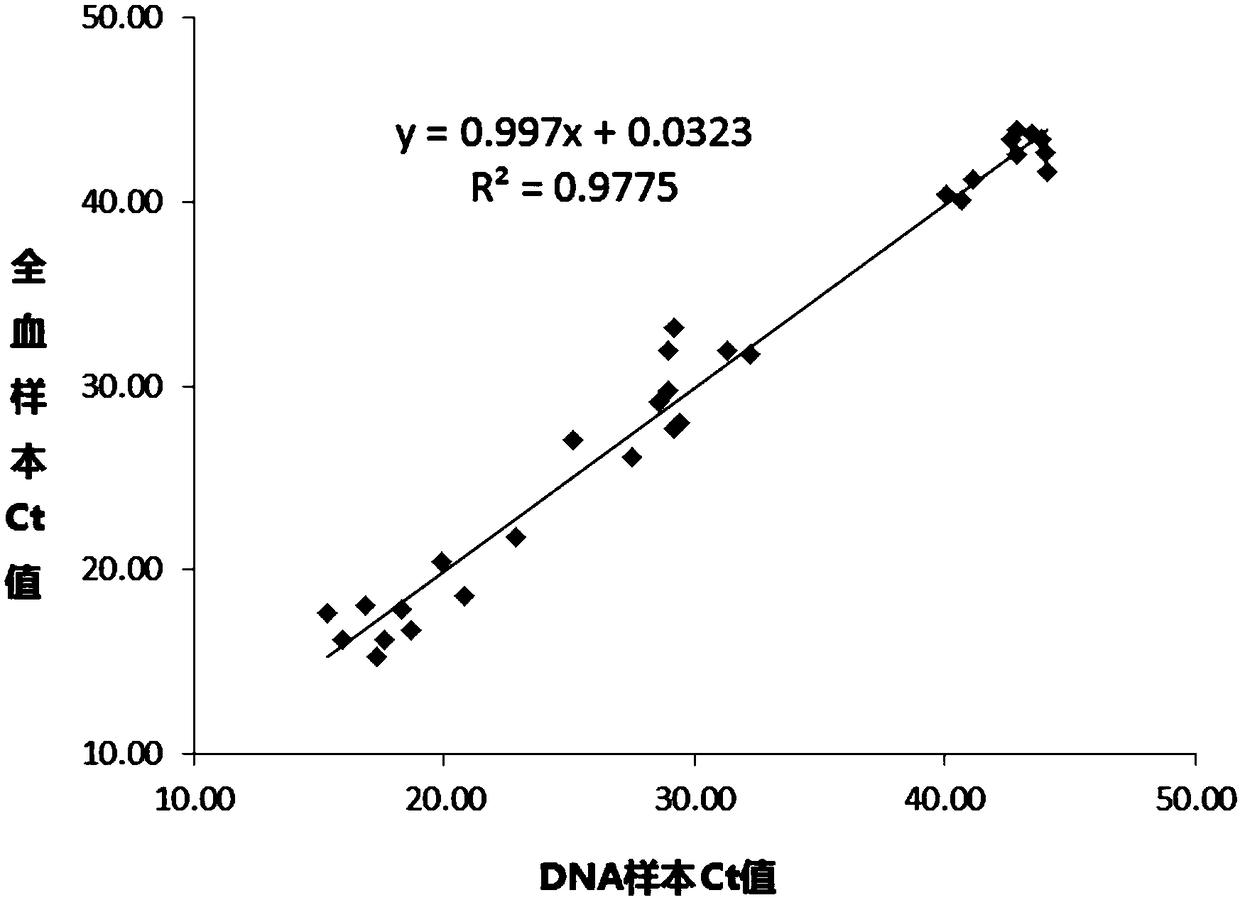
The invention discloses a kit for quantitative guidance of folic acid supplement dosage before and during pregnancy and application thereof. The kit comprises a specific primer pair, a specific fluorescent probe pair, a convention assembly and the like, wherein the specific primer pair and a specific fluorescent probe pair can simultaneously detect three single nucleotide polymorphism genotypes on a 5, 10-methylene tetrahydrofolate reductase (MTHFR) gene and a methionine synthase reductase (MTRR) gene, and the convention assembly is used to for fluorescent quantitative PCR detection. The kit of the invention can be used for the quantitative guidance of the folic acid supplement dosage before and during the pregnancy.
Owner:HAINAN ZHUJIAN BIOTECH
Pharmaceutical composition comprising progestogens and/or estrogens and 5-methyl- (6S)-tetrahydrofolate
The present invention relates to a pharmaceutical composition which may comprise progestogens, preferably drospirenone, estrogens, preferably ethinylestradiol and 5-methyl-(6S)-tetrahydrofolate, which may be employed as oral contraceptive and moreover prevents disorders caused by folate deficiency in the consumers, in particular cardiovascular disorders and, after conception of the embryo, congenital malformations caused by folate deficiency such as, for example, neural tube defects, ventricular valve defects, urogenital defects, and cleft lip, jaw and palate, without masking the symptoms of vitamin B12 deficiency, and at the same time even in the case of homozygous or heterozygous polymorphism of methylenetetrahydrofolate reductase facilitates unimpaired utilizability of the folate component 5-methyl-(6S)-tetrahydrofolate by the body and thus its biological activity for preventing the abovementioned congenital malformations caused by folate deficiency. In addition, a prolonged protective effect is maintained after discontinuation of the contraceptive.
Owner:SCHERING AG +1
Drug delivery systems (WAFER) for pediatric use
InactiveUS20120207836A1Improve complianceUnpleasant taste is effectivelyPowder deliveryBiocideAlkaline earth metalWater soluble
The present invention describes drug delivery compositions in the form of thin water-soluble films (wafers), which contain particles that comprise at least one active ingredient—which is not an estrogen and / or a progestin and / or an alkaline earth metal salt of 5-methyl-(6S)-tetrahydrofolate—and at least one protective agent. The protective agent provides effective taste-masking of the active ingredient due to limited release of the active ingredient in the mouth. The active ingredient is hence not absorbed via the buccal route, but rather via the enteral (per-oral) route. The particles contained in the wafer provided by the present invention have a particle size of below 40 μm thereby resulting in an acceptable sensation in the mouth while dissolving. Such wafers are especially suitable for pediatric use.
Owner:BAYER INTELLECTUAL PROPERTY GMBH
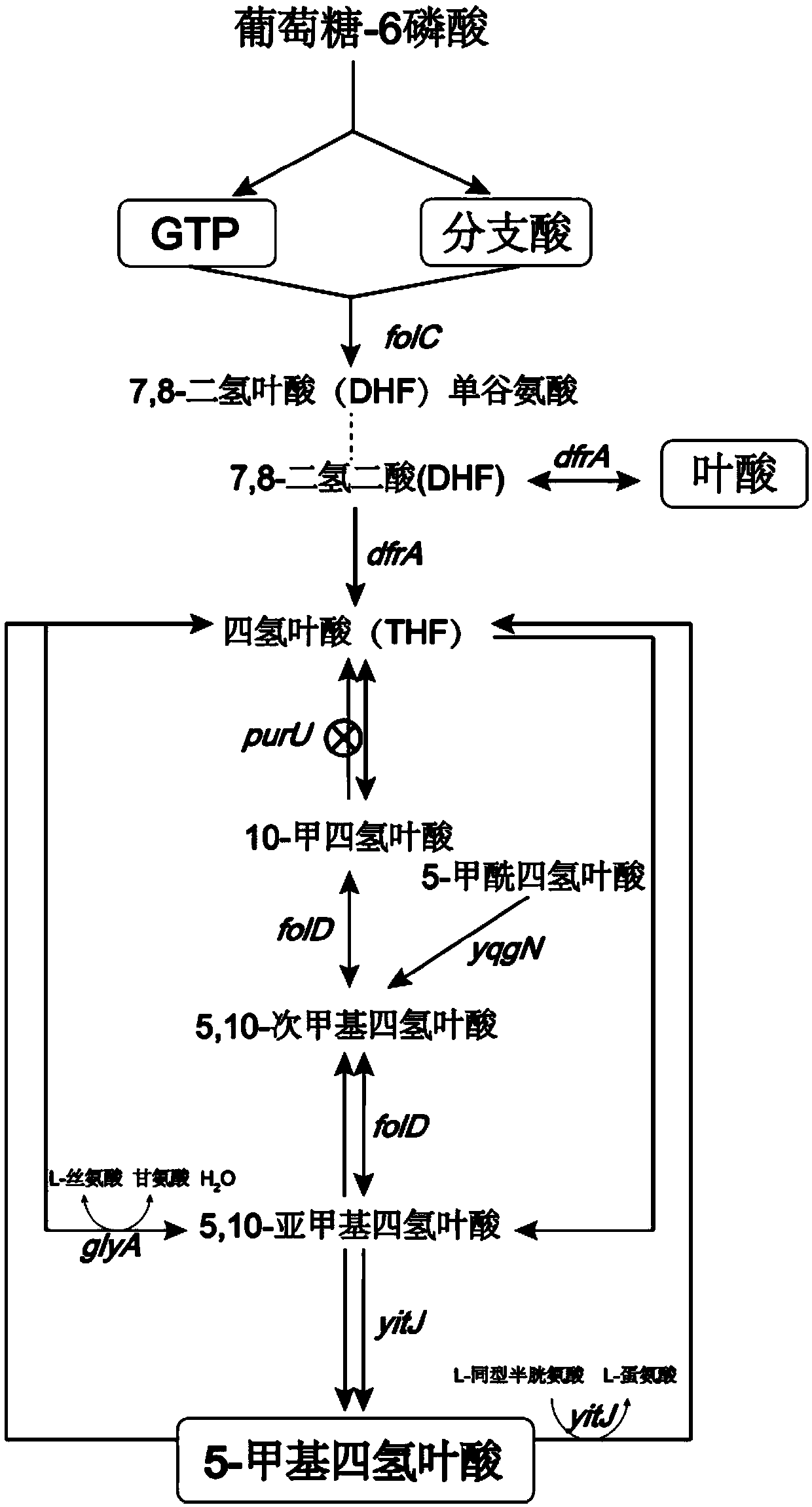
High-yield 5-methyl tetrahydrofolate recombinant bacillus subtilis and application thereof
ActiveCN109652351AEnhanced metabolic pathwaysIncrease productionBacteriaMicroorganism based processesDihydrofolate synthaseGene engineering
The invention discloses a high-yield 5-methyl tetrahydrofolate recombinant bacillus subtilis and an application thereof, and belongs to the field of genetic engineering. The high-yield 5-methyl tetrahydrofolate recombinant bacillus subtilis uses the bacillus subtilis 168 as the expression host; by over-expresseing 5,10-methylene tetrahydrofolate reductase coding gene metF, dihydrofolate reductasecoding gene dfrA, dihydrofolate synthase coding gene folC, knocking-out double function homocysteine S-methyltransferase / 5,10-methylene tetrahydrofolate reductase coding gene yitJ, formyltetrahydrofolate deformylase coding gene purU, the high-yield 5-methyl tetrahydrofolate recombinant bacillus subtilis obtains accumulation of 5-methyl tetrahydrofolate bacillus subtilis gene engineering bacteria;the yield of 5-methyl tetrahydrofolate reached 952.05 ug / L, and the conversion cell dry weight is 342.40ug / g DCW.
Owner:JIANGNAN UNIV
Nutritional supplement for pregnant females
Preeclampsia and intrauterine growth restriction in a pregnant female mammal are prevented or decreased in severity by administering thereto a combination of a vitamin compound containing B1, Folic Acid (or active form 5-Methyl-Tetrahydrofolate i.e.; Metafolin®), B 6 (Pyridoxine or active form Pyridoxine 5-Phosphate P5P), B 12, Ascorbic Acid, Selenium, Zinc, Co-enzyme Q 10 and N-Acytyl Cysteine, Lycopene, optionally in further combination with Melatonin and / or Vitamin E or / and ASA 81 mg both of later, are cyclooxygenase inhibitors, a PGI.sub.2-mimetic, a thromboxane (TXA.sub.2) inhibitor, a compound possessing TXA.sub.2-agonistic and TXA.sub.2-inhibiting properties, a compound possessing TXA.sub.2-antagonistic and PGI.sub.2-mimetic activities, and a TXA.sub.2 antagonist.
Owner:MUENCH MICHAEL V +2
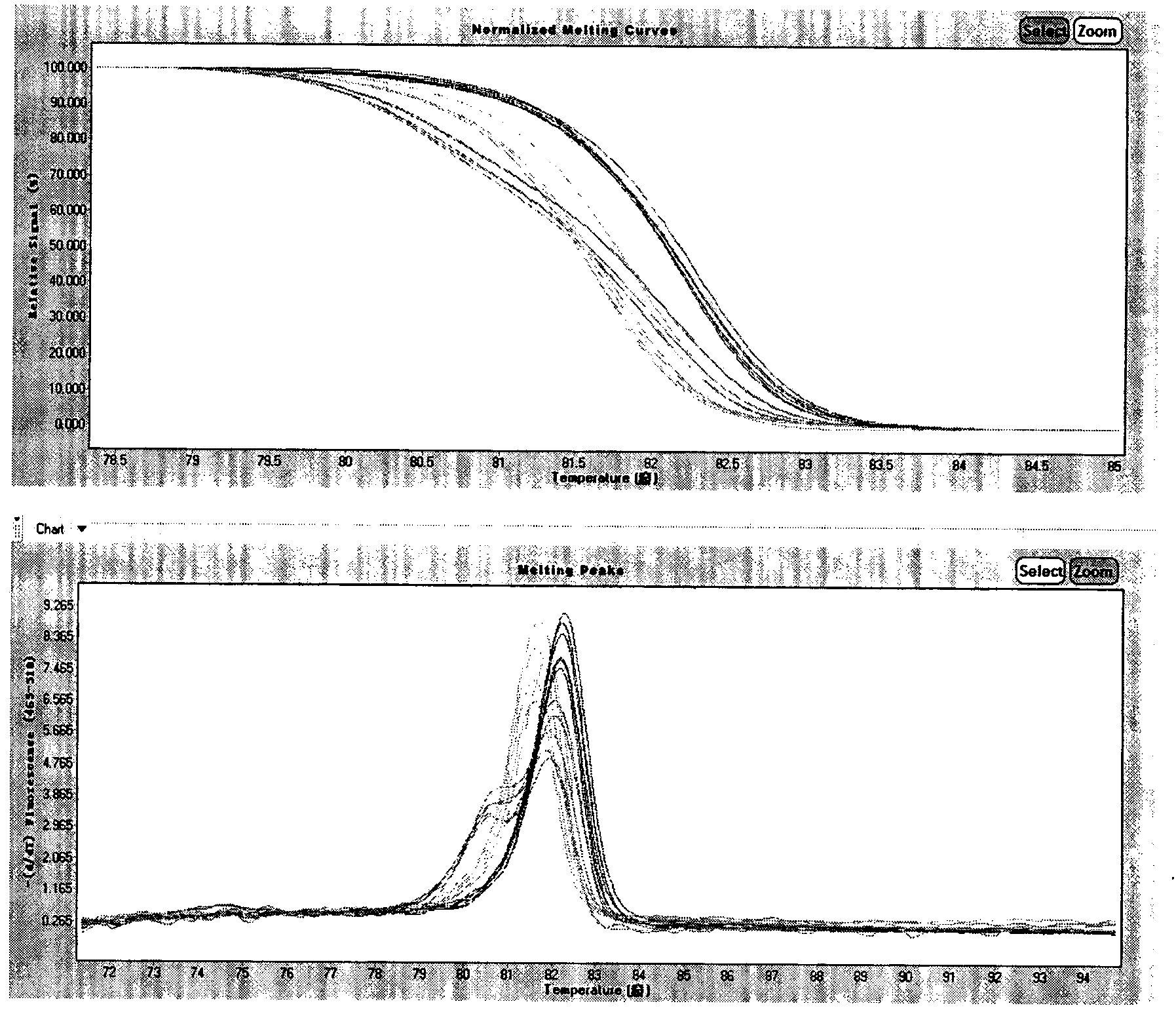
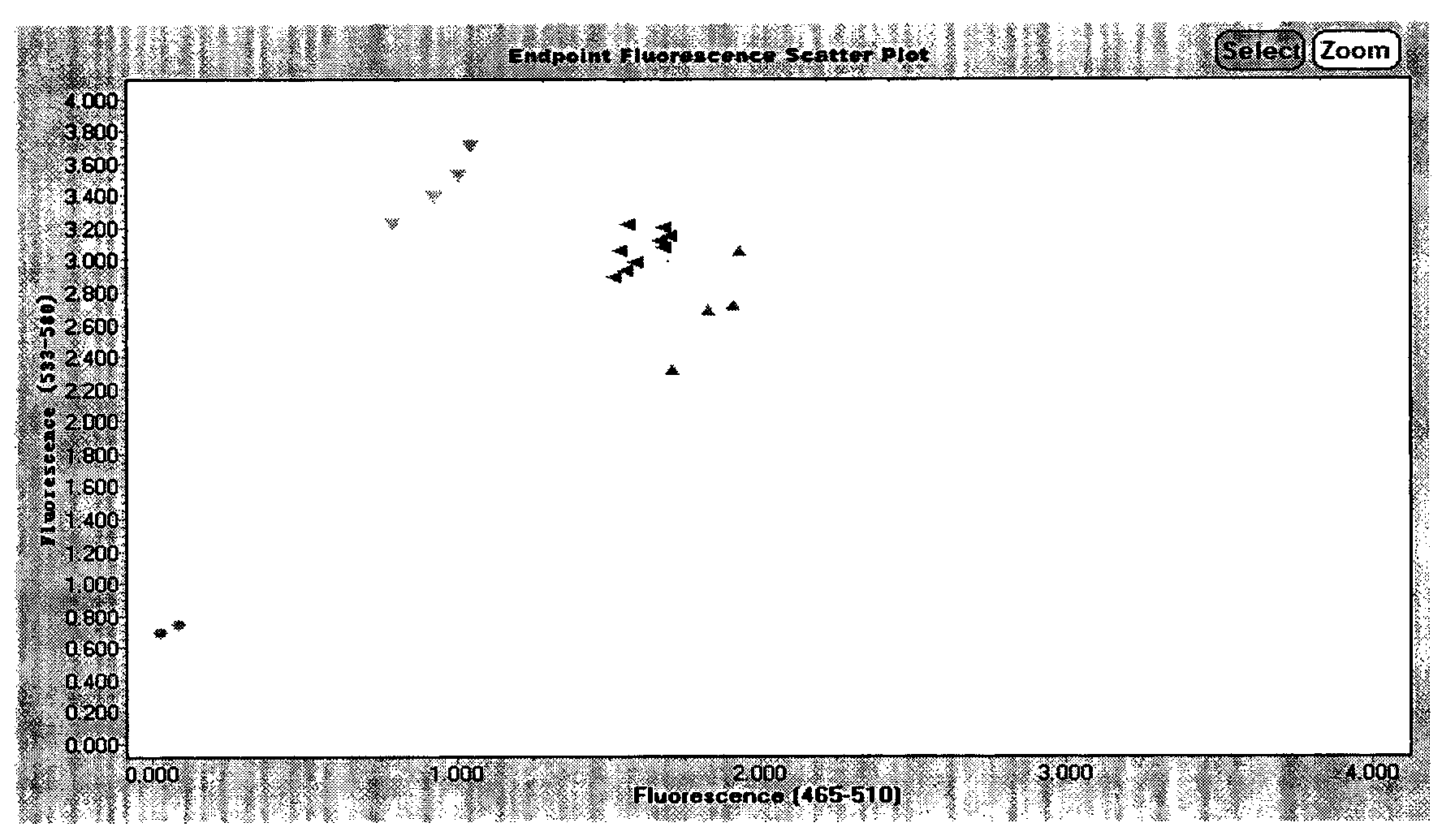
Kit and method for detecting MTHFR (methylene tetrahydrofolate reductase) and MTRR (methionine synthase reductase) gene polymorphism simultaneously with molecular beacon probes and melting curve
InactiveCN107828870APolymorphism detection is accuratePolymorphism detection stableMicrobiological testing/measurementDNA/RNA fragmentation(Methionine synthase) reductaseBiology
The invention relates to an application of molecular beacon probes and a melting curve technology in detection of gene polymorphism, in particular to a method for detecting MTHFR (methylene tetrahydrofolate reductase) and MTRR (methionine synthase reductase) gene polymorphism simultaneously, and further relates to amplification primers of MTHFR and MTRR genes, the molecular beacon probes and a kitcontaining the amplification primers and the molecular beacon probes. The method is simple and fast to operate, good in specificity, high in sensitivity, accuracy and flux and low in detection cost and has broad application prospect. According to the method, PCR amplification and detection are performed synchronously, the overall detection process does not require uncovering operation, so that detection period is shortened and detection efficiency is improved while detection cost is saved, and besides, risk of false positive caused by PCR product pollution is reduced.
Owner:沈阳迪安医学检验所有限公司
Human MTHFR (Methylene Tetrahydrofolate Reductase) and/or MTRR (Methylenetetrahydrofolate Reductase) gene polymorphism investigation kit
ActiveCN107988353AEasy to operateReduce pollutionMicrobiological testing/measurementMgb probePlasmid
The invention discloses a kit for investigating polymorphism of an MTHFR (Methylene Tetrahydrofolate Reductase) and / or MTRR (Methylenetetrahydrofolate Reductase) gene on the basis of a Taqman-MGB probe. The kit comprises a primer group and a probe, wherein the primer group is at least one selected from the following three groups: primers of SEQ ID NO.1 and SEQ ID NO.4 for an MTHFR gene at a C677Tsite, primers of SEQ ID NO.7 and SEQ ID NO.10 for an MTRR gene at an A1298C site, primers of SEQ ID NO.13 and SEQ ID NO.14 for an MTRR gene at an A66G site, primers of SEQ ID NO.22 and SEQ ID NO.19, primers of SEQ ID NO.29 and SEQ ID NO.15, and primers of SEQ ID NO.33 and SEQ ID NO.30. The kit disclosed by the invention has the advantages that three mutation sites can be detected simultaneously, high sensitivity is achieved, plasma as low as 10copies can be accurately detected, and oral cavity swabs which are too long in preservation time or relatively low in concentration can be still accurately detected.
Owner:SUREXAM BIO TECH
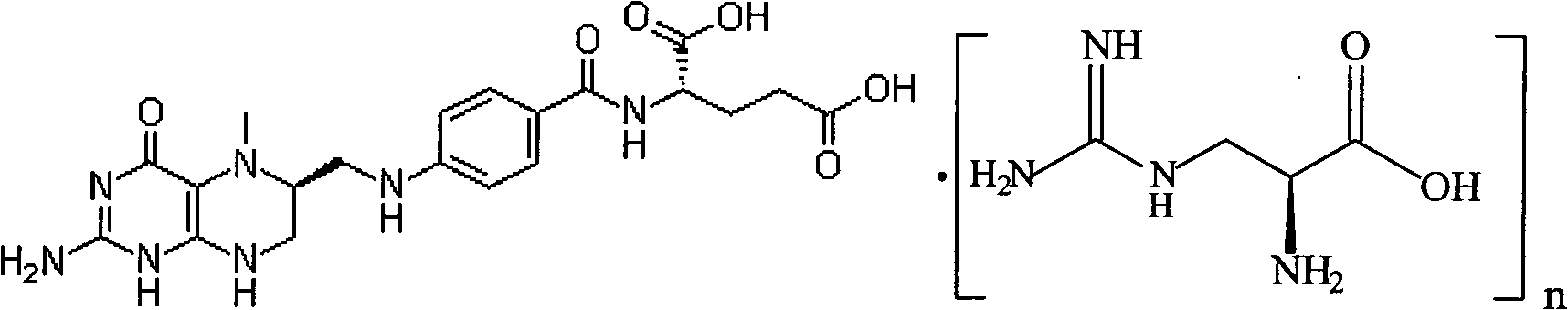
Preparation method of L-5-methyl tetrahydrofolate amino acid salt
The invention discloses a preparation method of an L-5-methyl tetrahydrofolate amino acid salt. The preparation method comprises the following steps of suspending L-5-methyl calcium tetrahydrofolate in alcohol under nitrogen gas flow; slowly adding hydrochloric amino acid or free amino acid dissolved in water into L-methyl calcium tetrahydrofolate suspension; sufficiently stirring the mixture, keeping at 0 DEG C-20 DEG C, furtherstirring for 8 hours, regulating the pH value to 6.0-6.8; dropwise adding absolute ethyl alcohol or acetone, stewing for 24 hours; filtering, washing by cold alcohol / water, and drying in vacuum at 20 DEG C-40 DEG C to obtain the L-5-methyl tetrahydrofolate amino acid salt (for example L-5-methyl etrahydrofolate L-arginine salt).
Owner:NAN JING RHINE PHARM TECH
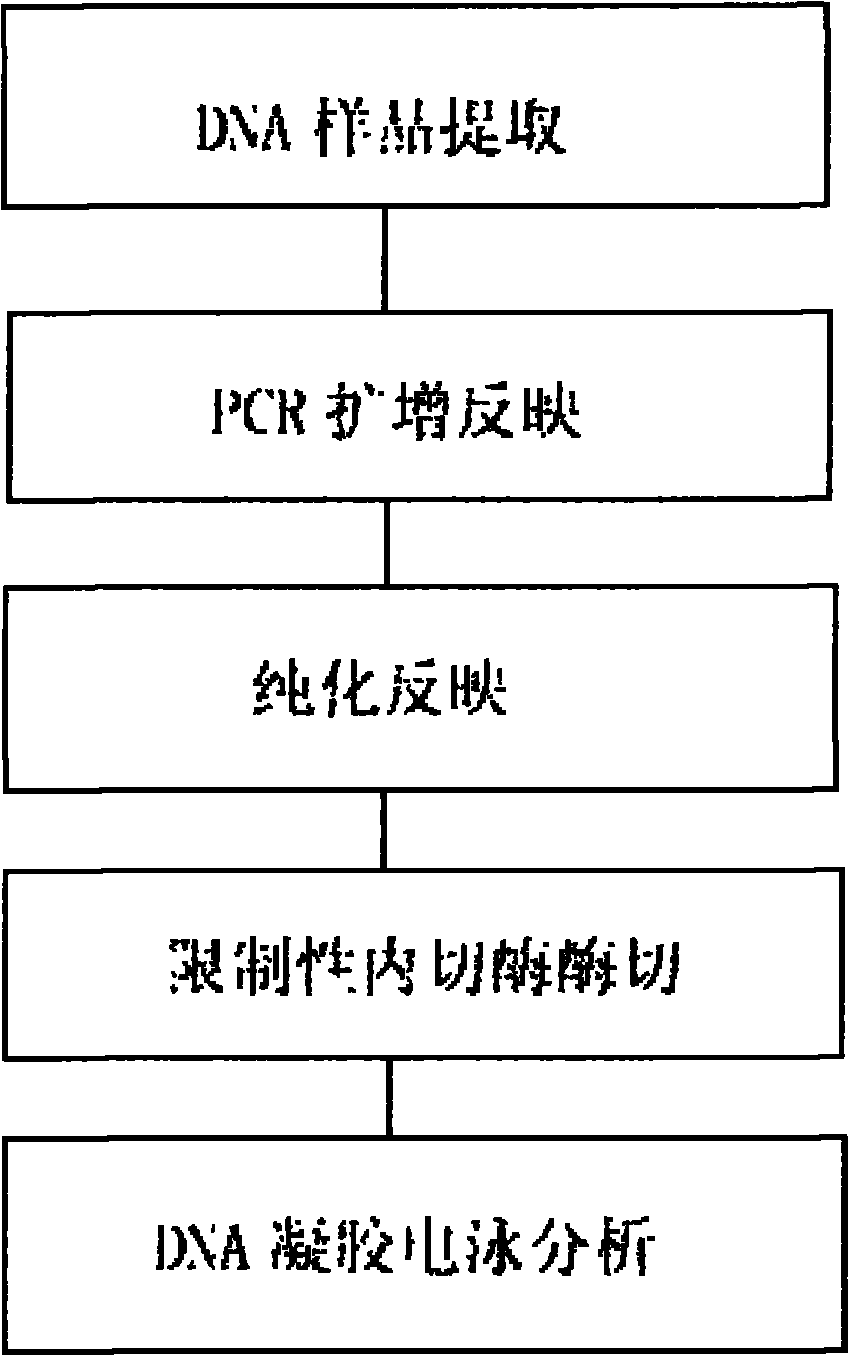
Gene noninvasive detection kit for preventing neural tube defects of newborns
The invention provides a gene noninvasive detection kit for preventing neural tube defects of newborns. The kit comprises specific primers, DNA sequencing primers, a PCR (Polymerase Chain Reaction) reaction assembly, a PCR product purification assembly, a DNA sequencing reaction assembly and the like, wherein the specific primers are used for detecting No. rs1801133 SNP locus (MTHFR C677T) and No. rs1801131 SNP locus (MTHFR A1298C) on 5, 10-methylene tetrahydrofolate reductase (MTHFR) and No. rs1801394 SNP locus (MTRR A66G) on 5, 10-methylene methyl transferase reductase (MTRR). The kit evaluates the risk level of pregnant women giving birth to infants with neural tube defects by detecting the genotype of a single nucleotide polymorphism locus closely related to the main genetic factor causing newborn defects, namely female folic acid metabolism ability, and the pregnant women are individually instructed to supplement folic acid according to the gene detection result of each client. The method provided by the invention accords with the situation of our country, oral mucosa cell sampling is adopted as a sampling method which is painless and noninvasive, and cross infection is avoided. The sequencing detection results are accurate and reliable, expensive imported special instruments are not needed, and the method is easy to popularize and spread.
Owner:解码(上海)生物医药科技有限公司
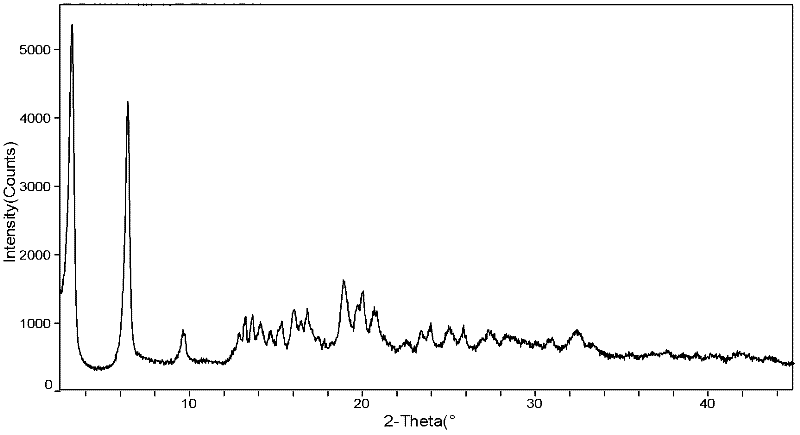
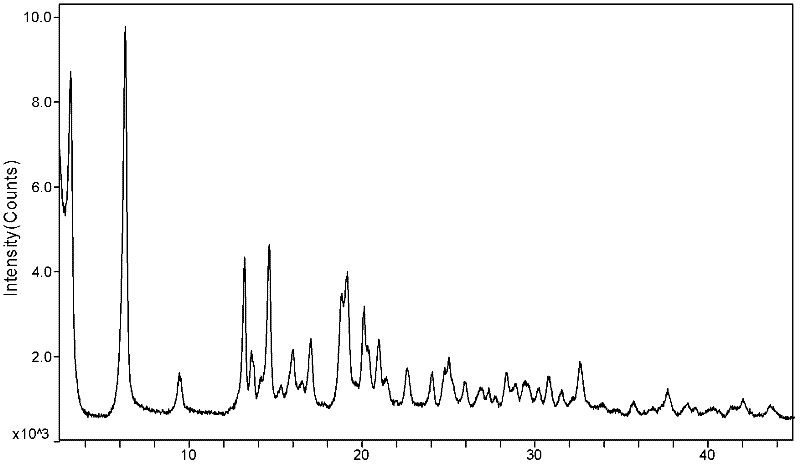
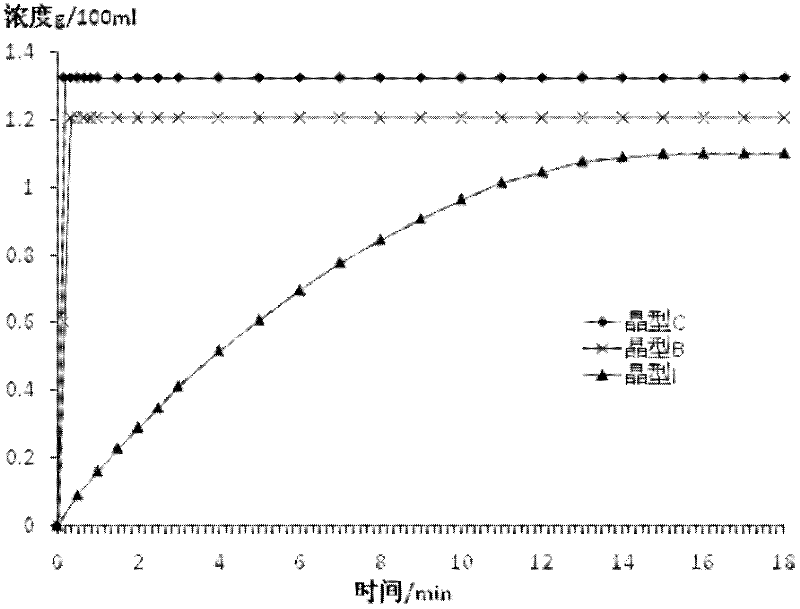
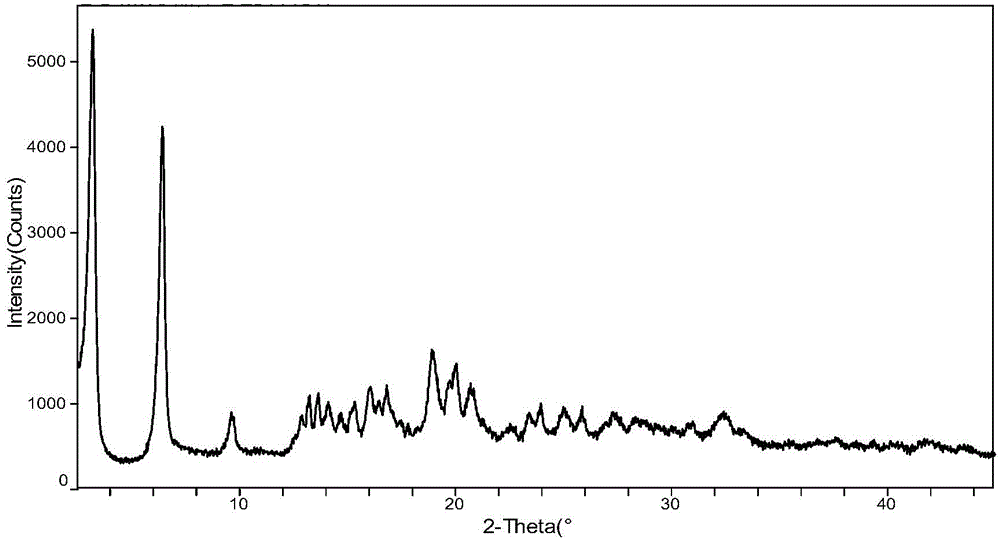
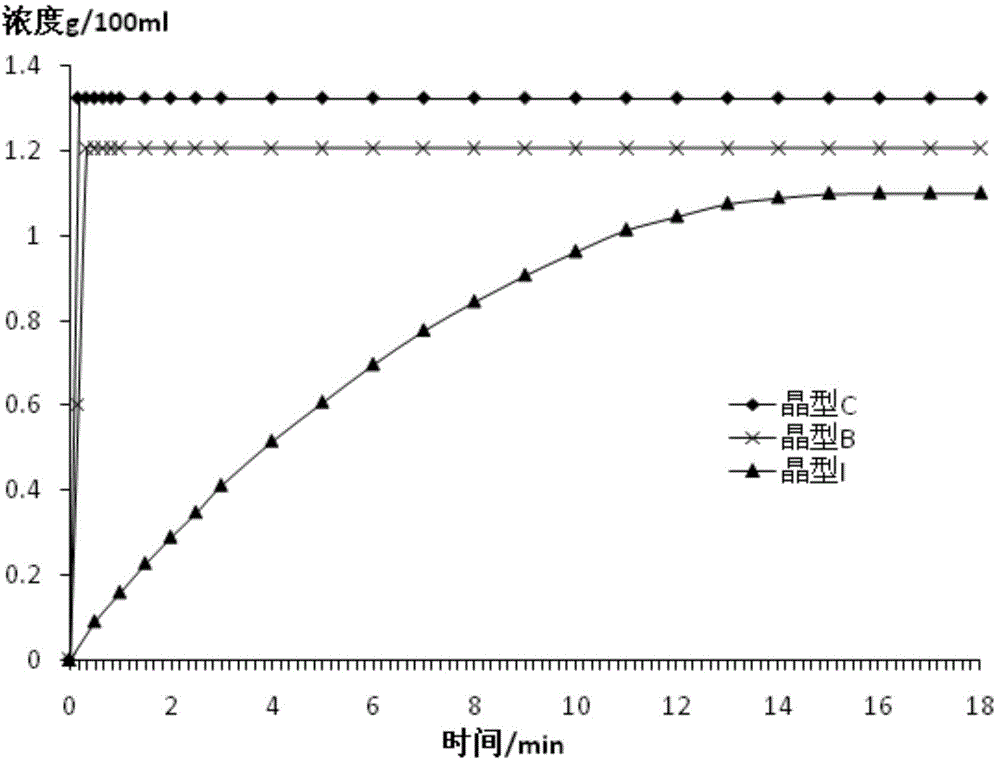
(6S)-5-methyl tetrahydrofolate crystal form and preparation method thereof
ActiveCN102584826AGood chemical stabilityHigh purityOrganic active ingredientsNervous disorderX-rayMethyl group
The invention discloses a (6S)-5-methyl tetrahydrofolate crystal form and a preparation method thereof. The crystal form is a B-type (6S)-5-methyl calcium tetrahydrofolate crystal form, and diffraction peaks exist at the positions where 2theta angle is 3.2+ / -0.2 and 18.9+ / -0.2 in an X-ray diffraction map of the B-type crystal form; or the crystal form is a C-type (6S)-5-methyl calcium tetrahydrofolate crystal form, and diffraction peaks exist at the positions where 2theta angle is 6.3+ / -0.2 and 19.2+ / -0.2 in the X-ray diffraction map of the C-type crystal form. The B-type (6S)-5-methyl calcium tetrahydrofolate crystal form and the C-type (6S)-5-methyl calcium tetrahydrofolate crystal form disclosed by the invention has the advantages of excellent physicochemical property, good stability, high purity, good repeatability and the like and are suitable for industrial large-scale preparation.
Owner:LIANYUNGANG JINKANG HEXIN PHARMA CO LTD
Application, detection method and kit of MTHFR (Methylene Tetrahydrofolate Reductase) gene SNP (Single Nucleotide Polymorphism) guide folic acid and related nutrient element intake
InactiveCN101864479ATimely and accurate nutritional intake guidancePrevent physical defectsMicrobiological testing/measurementFolate MetabolismPhysiology
The invention discloses application, a detection method and a kit of SNP of an METHFR gene guiding folic acid and related nutrient element intake. Because of providing different positive locuses of SNP gene types of folic acid metabolic capacity and related nutrient element intake, the reason generating impediment thereof is found, and the guidance for guiding individuals, especially pre and post natal women, to take folic acid and intake related nutrients is provided.
Owner:吴奇涵 +2
Kit used for detecting susceptibility to femoral head necrosis
InactiveCN102108413AImprove accuracyImprove throughputMicrobiological testing/measurementVascular endotheliumNucleotide
The invention discloses a kit used for detecting susceptibility to femoral head necrosis. The kit detects six genes which have close relation with the femoral head necrosis, namely a vascular endothelial growth factor (VEGF) gene of vascular endothelial factors, a coagulation factor V (FV) gene of coagulation factors, a methylene tetrahydrofolate reductase (MTHFR) gene, a pamoxonase 1 (PON1) gene of lipid metabolism related gene, an apolipoprotein A1 (APOA1) gene and an apolipoprotein B (APOB) gene. The kit detects a group of genes and loci related to the susceptibility to the femoral head necrosis through specific primers and probes by combining a single nucleotide extension technology with a microarray chip technology, can clearly judge whether examinees carry femoral head necrosis susceptibility genes and screens a group susceptible to the femoral head necrosis from the examinees, so as to make the group change bad living habits and fulfill the aim of preventing the femoral head necrosis.
Owner:UNION STEMCELL & GENE ENG +1
Stabilised particles comprising 5-methyl-(6s)-tetrahydrofolate
The present invention relates to small stabilised particles comprising a crystalline form of an alkaline earth metal salt of 5-methyl-(6S)-tetrahydrofolate and at least one protective agent. Such particles confer stability to the alkaline earth metal salt of 5-methyl-(6S)-tetrahydrofolate, and is conveniently incorporated in unit dosage forms, such as wafers.
Owner:BAYER PHARMA AG
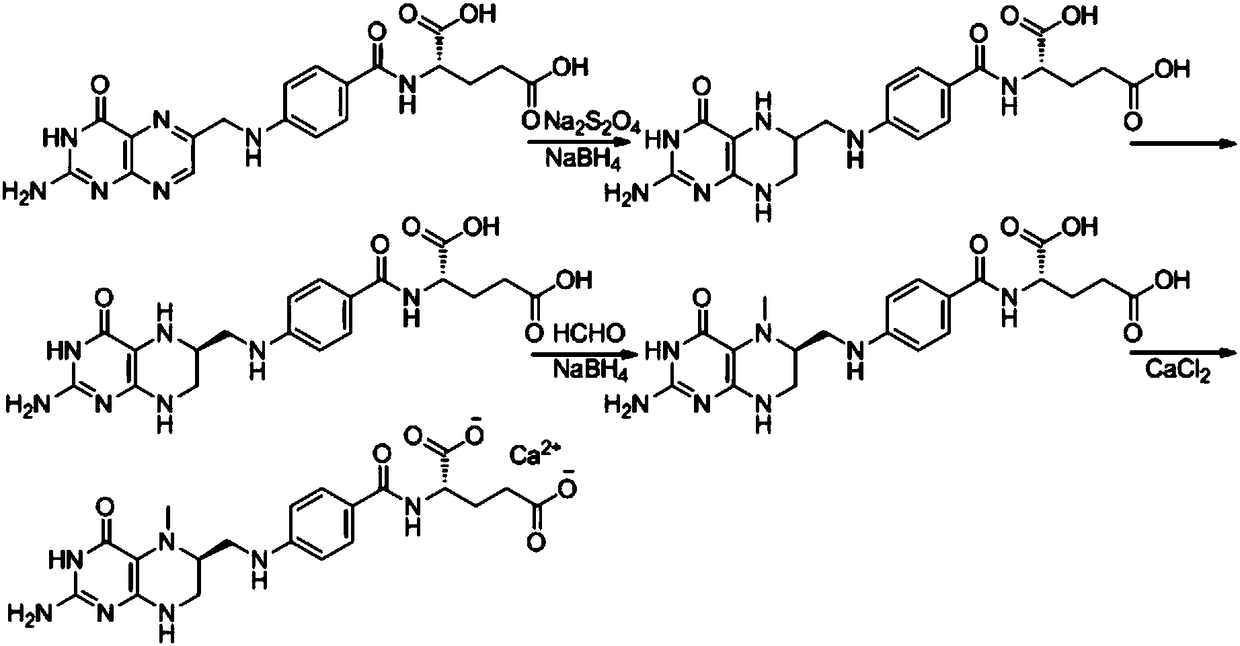
Preparation method of L-5-calcium methyltetrahydrofolate
ActiveCN108164531AReduce usageRaw materials are cheap and easy to getOrganic chemistry methodsHydrogenWastewater
The invention discloses a preparation method of L-5-calcium methyltetrahydrofolate. The preparation method comprises the following steps of (1) adopting folic acid as a raw material, and reducing to preparing an intermediate 6-(R,S)-tetrahydrofolic acid; (2) carrying out chiral resolution on the 6-(R,S)-tetrahydrofolic acid, and obtaining an intermediate 6-S-tetrahydrofolate; (3) carrying out methylation on the 6-S-tetrahydrofolate to obtain an intermediate L-5-methyltetrahydrofolate; (4) reacting the L-5-methyltetrahydrofolate and salt containing calcium to obtain the L-5-calcium methyltetrahydrofolate. According to the method provided by the invention, the problem that hydrogen, wastewater and waste residues are easily generated by using a large number of sodium borohydride is avoided.
Owner:无锡紫杉药业股份有限公司
Kit and detection method of folic acid metabolic capability and genotyping as well as application of kit
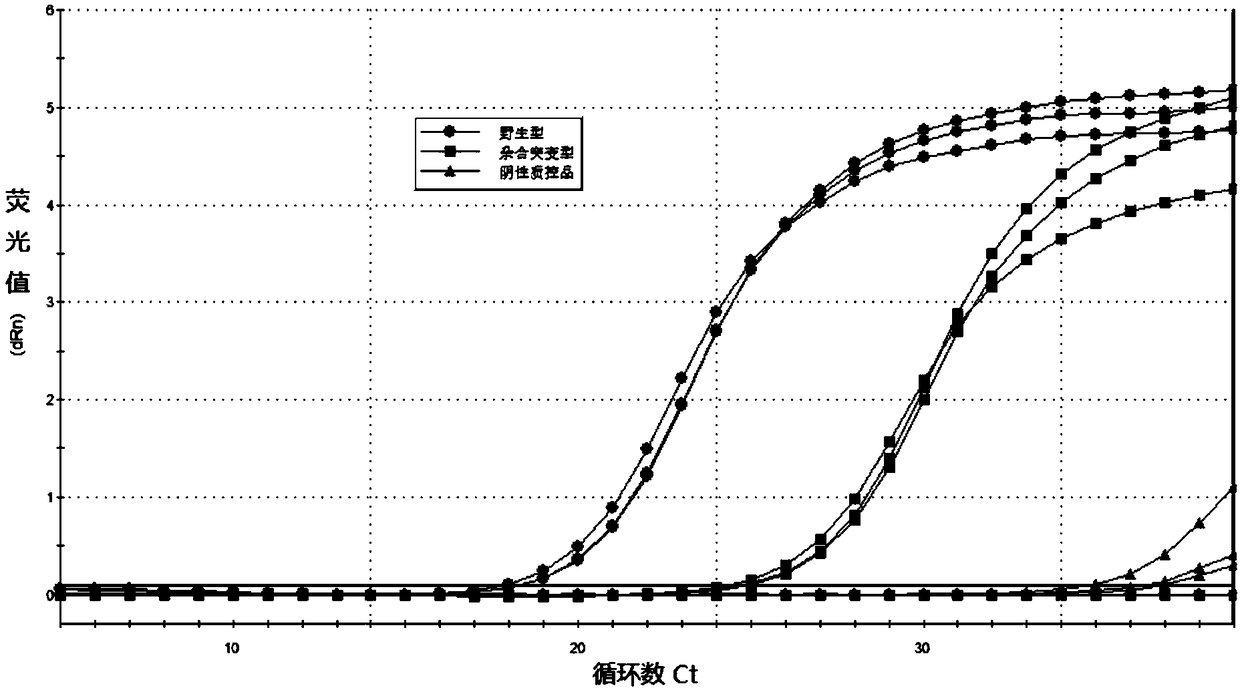
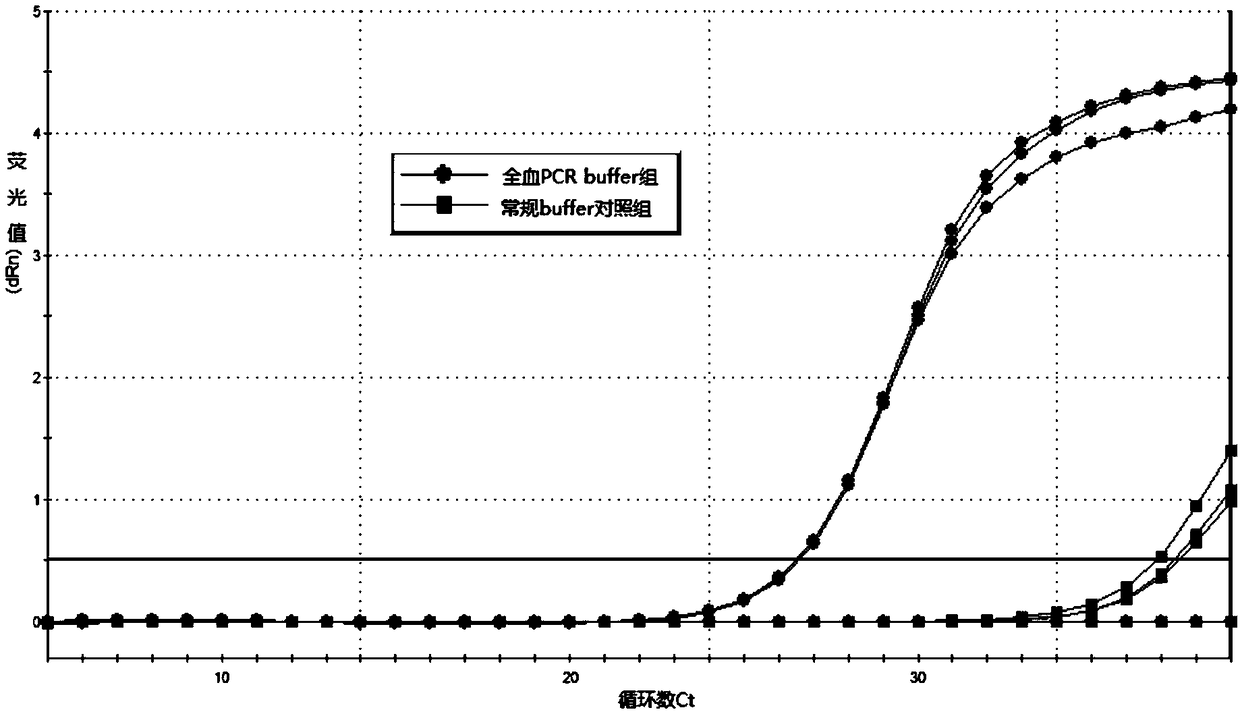
PendingCN108251510AAvoid the extraction stepEliminate factors that inhibit the PCR reactionMicrobiological testing/measurementWild type(Methionine synthase) reductase
The invention relates to a folic acid metabolic capability and genotyping detection technology and in particular relates to a kit and a detection method of a folic acid metabolic capability and genotyping as well as application of the kit. The kit comprises specific primers aiming at 677C>T mutation and 1298A>C mutation of an MTHFR (Methylene Tetrahydrofolate Reductase) gene, and 66A>G mutation ofan MTRR (Methionine Synthase Reductase) gene, and a specific mutation detection probe, RT-PCR (Reverse Transcription-Polymerase Chain Reaction) buffer, an RT-PCR reaction Taq enzyme, sterile purifiedwater, a negative quality control product, a wild type quality control product, a mutant quality control product and a packaging box for separating and packaging a reagent bottle or pipe. The detection method of the folic acid metabolic capability and the genotyping, provided by the invention, has the advantages of strong specificity, high sensitivity, small pollution, simplicity and rapidness inoperation, high safety performance and the like; a detection result has relatively good accuracy and repeatability; the detection method is especially suitable for directly taking whole blood as a detection sample and related gene mutation detection is directly carried out; a genome DNA (Deoxyribonucleic Acid) template does not need to be extracted. The folic acid metabolic capability can be accurately judged and individualized folic acid oral administration and supplementing dosages are provided; the kit and the detection method have important value.
Owner:PRO MED BEIJING TECH
Nucleotide primer set and nucleotide probe for detecting genotype of methylene tetrahydrofolate reductase (MTHFR)
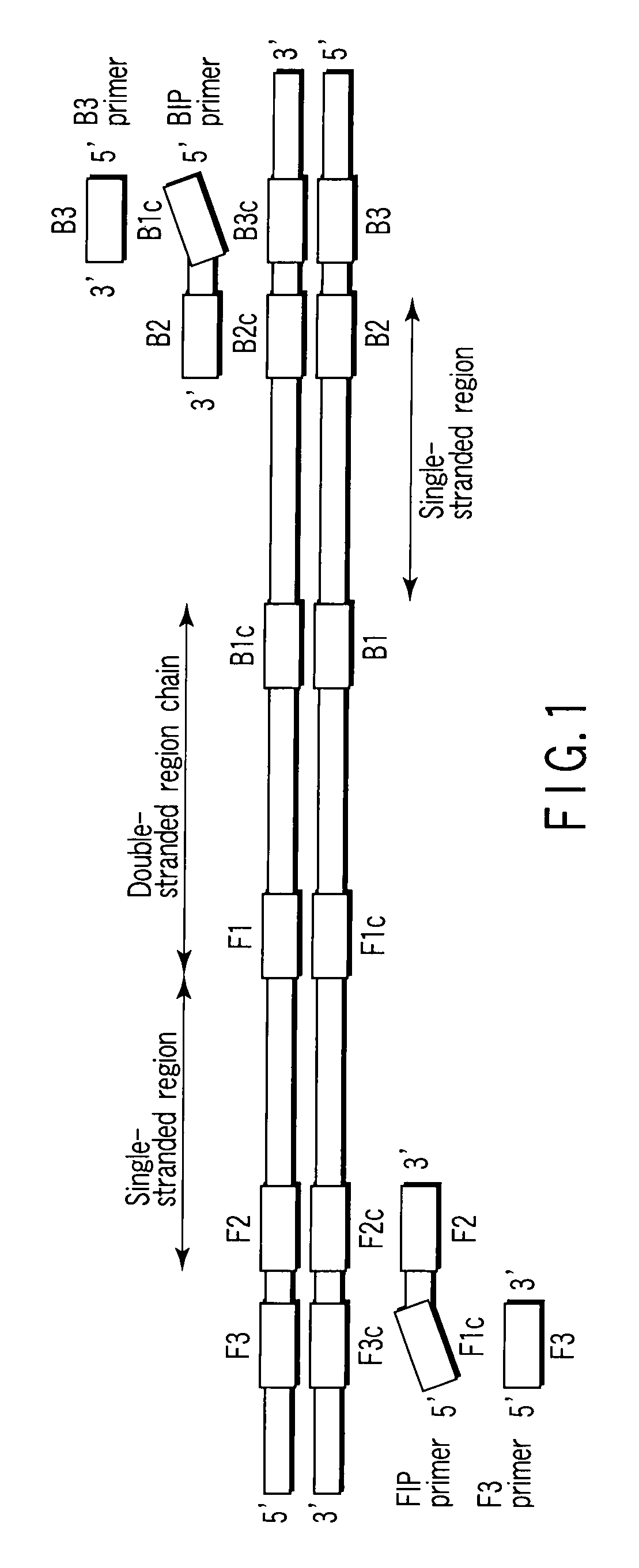
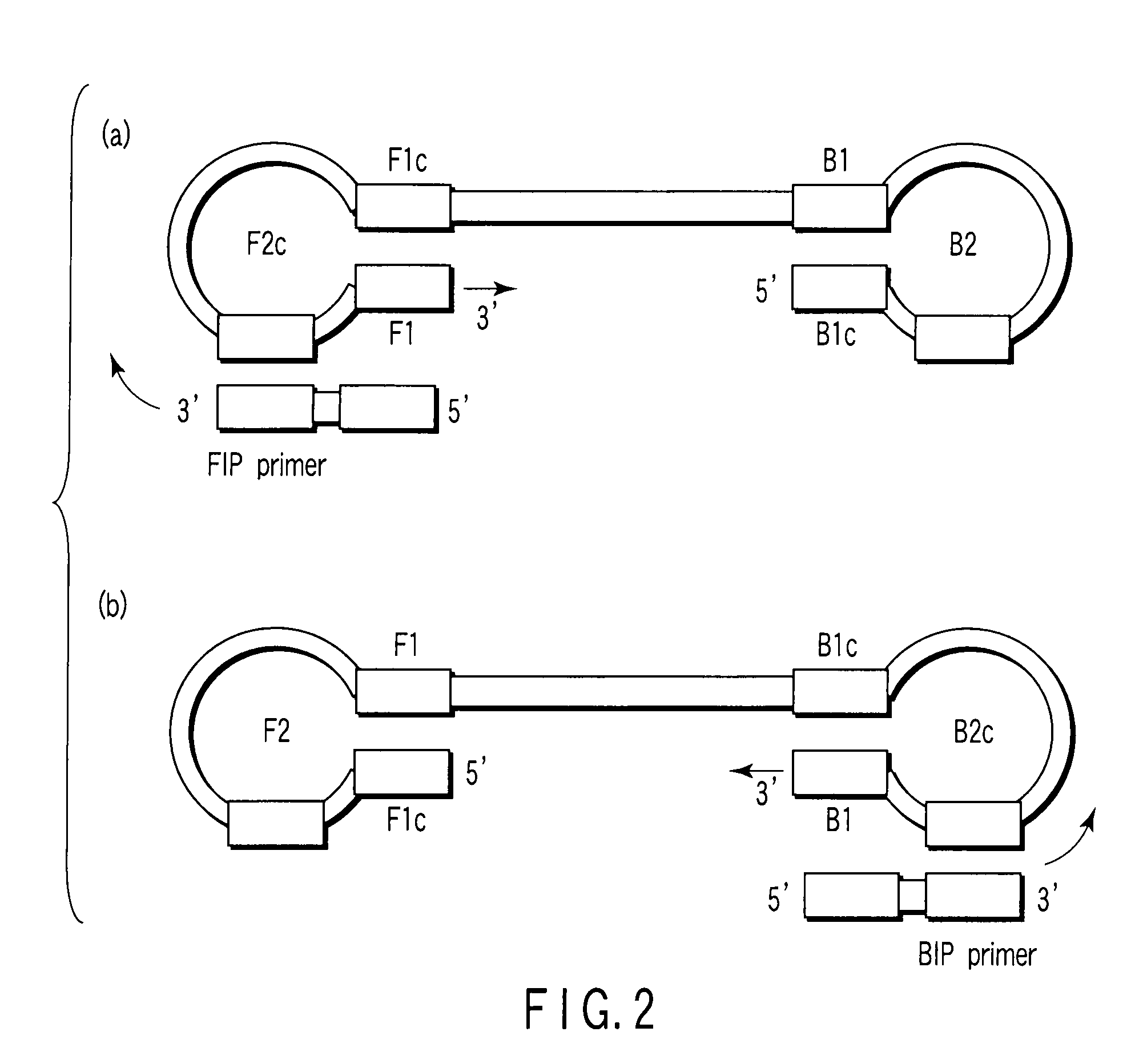
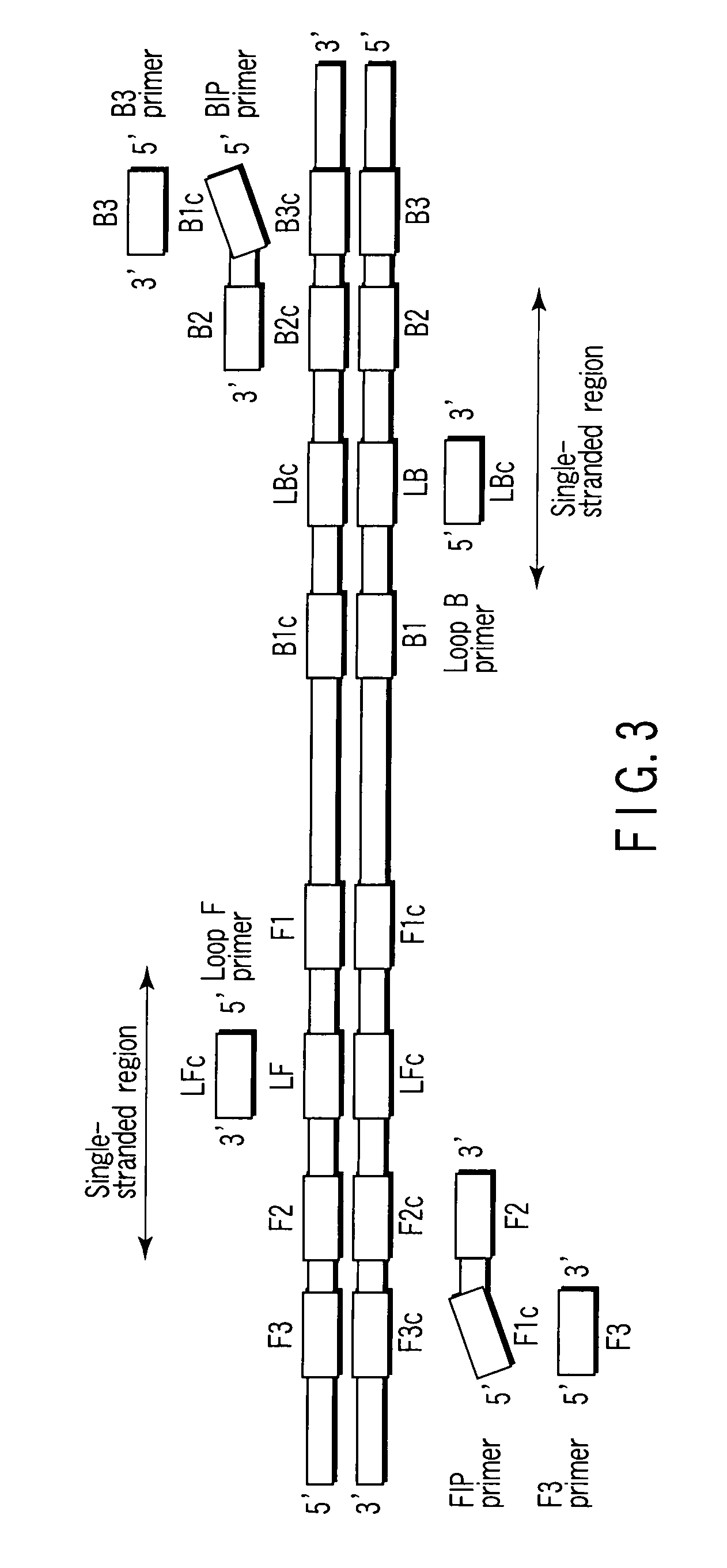
There is provided is a nucleotide primer set for LAMP amplification used for detecting genotypes of single-nucleotide polymorphisms C677T and A1298C of an MTHFR gene. There is also provided a nucleotide probe for detecting an amplification product amplified by the primer set according to the present invention. There is also provided a method of detecting the genotypes of the single-nucleotide polymorphisms C677T and A1298C in the MTHFR gene, by using the primer set according to the present invention.
Owner:KK TOSHIBA
Kit for detecting individual folate metabolism disorder
InactiveCN103667435AMicrobiological testing/measurementFolate Metabolism(Methionine synthase) reductase
The invention discloses a kit for detecting an individual folate metabolism disorder. The kit comprises a specific primer pair and SYBRGreenI fluorochrome for simultaneously detecting rs1801133# SNP site on a 5,10-methylene tetrahydrofolate reductase gene, rs1801131# SNP on a 5,10-methylene tetrahydrofolate reductase gene and rs1801394# SNP site on a methionine synthase reductase gene, a conventional component for real-time quantitative polymerse chain reaction (PCR) detection. The kit disclosed by the invention evaluates the metabolic capability of individual folate by simultaneously detecting single nucleotide polymorphism site genotype of the 5,10-methylene tetrahydrofolate reductase gene and the methionine synthase reductase gene of folate metabolism.
Owner:浙江爱易生物医学科技有限公司
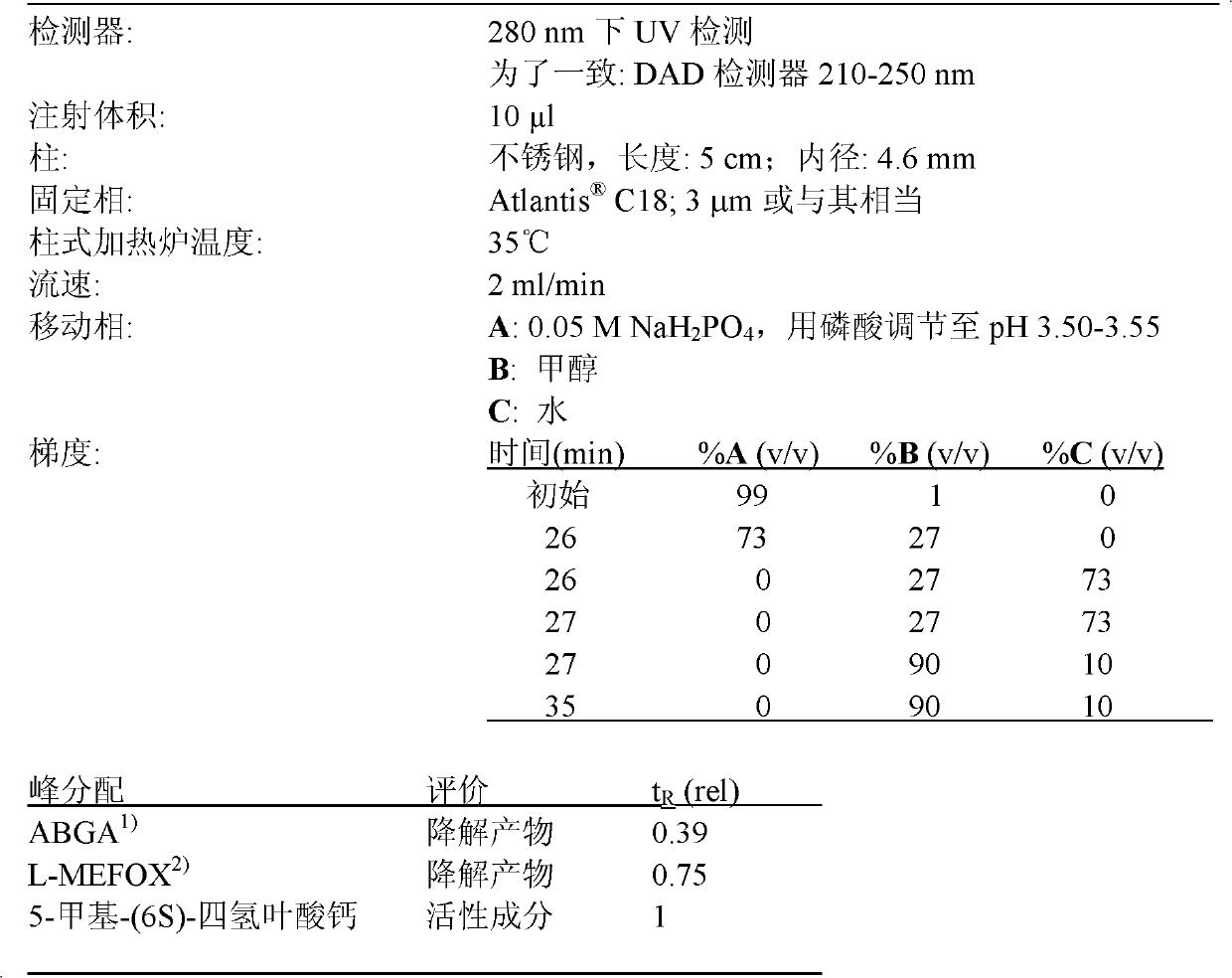
Stable pharmaceutical composition of (6S)-5-methyl-calcium tetrahydrofolate
InactiveCN104490887AQuick and reliable releaseImprove stabilityOrganic active ingredientsPharmaceutical non-active ingredientsMethyl groupClelands Reagent
The invention discloses a stable pharmaceutical composition, which contains (6S)-5-methyl-calcium tetrahydrofolate crystal and / or pharmaceutically acceptable reducing substances, and pharmaceutically acceptable auxiliary materials. Specifically, the reducing substances can protect (6S)-5-methyl-tetrahydrofolic acid and its salt from being oxidized, and can be selected from vitamin C and its salt, isovitamin C and its salt, mercaptoethanol, cysteine, mercaptoethyl sulfonic acid, dithiothreitol, reduced glutathione, and lipoic acid. The stable pharmaceutical composition provided by the invention has good stability during processing and storage, the drug risk caused by degradation can be avoided, and at the same time fast and reliable release of (6S)-5-methyl-tetrahydrofolic acid in the composition can be ensured.
Owner:LIANYUNGANG JINKANG HEXIN PHARMA CO LTD
Nucleotide primer set and nucleotide probe for detecting genotype of methylene tetrahydrofolate reductase (MTHFR)
There is provided is a nucleotide primer set for LAMP amplification used for detecting genotypes of single-nucleotide polymorphisms C677T and A1298C of an MTHFR gene. There is also provided a nucleotide probe for detecting an amplification product amplified by the primer set according to the present invention. There is also provided a method of detecting the genotypes of the single-nucleotide polymorphisms C677T and A1298C in the MTHFR gene, by using the primer set according to the present invention.
Owner:KK TOSHIBA
Pharmaceutical composition comprising progestogens and/or estrogens and 5-methyl-(6S)-tetrahydrofolate
The present invention relates to a pharmaceutical composition which comprises progestogens, preferably drospirenone, estrogens, preferably ethinylestradiol and 5-methyl-(6S)-tetrahydrofolate, can be employed as oral contraceptive and moreover prevents disorders caused by folate deficiency in the consumers, in particular cardiovascular disorders and, after conception of the embryo, congenital malformations caused by folate deficiency such as, for example, neural tube defects, ventricular valve defects, urogenital defects, and cleft lip, jaw and palate, without masking the symptoms of vitamin B12 deficiency, and at the same time even in the case of homozygous or heterozygous polymorphism of methylenetetrahydrofolate reductase facilitates unimpaired utilizability of the folate component 5-methyl-(6S)-tetrahydrofolate by the body and thus its biological activity for preventing the abovementioned congenital malformations caused by folate deficiency. In addition, a prolonged protective effect is maintained after discontinuation of the contraceptive.
Owner:MERCK & CIE KG +1
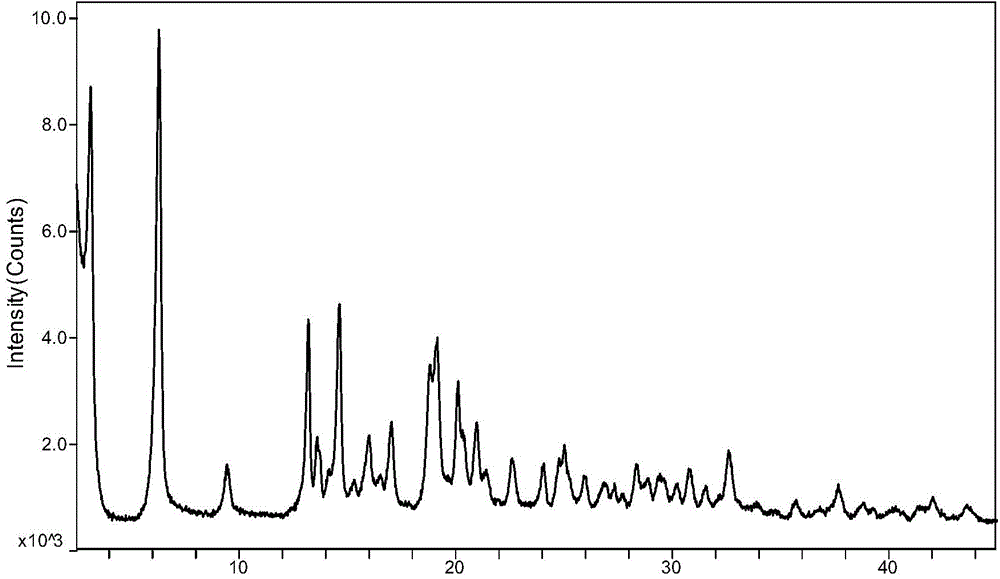
(6S)-5-methyl tetrahydrofolate crystal form and preparation method thereof
ActiveCN104557937AGood chemical stabilityHigh purityOrganic active ingredientsNervous disorderX-rayMethyl group
The invention discloses a (6S)-5-methyl tetrahydrofolate crystal form and a preparation method thereof. The crystal form is a type C calcium (6S)-5-methyl tetrahydrofolate crystal form; the X-ray diffraction spectrum has diffraction peaks at angles 2 theta, namely 6.3+ / -0.2 and 19.2+ / -0.2. The type C calcium (6S)-5-methyl tetrahydrofolate crystal form has the advantages of excellent physicochemical properties, good stability, high purity, good reproducibility, better suitability for industrial large-scale preparation, and the like.
Owner:LIANYUNGANG JINKANG HEXIN PHARMA CO LTD
Detection method and kit for coronary heart disease susceptibility loci rs1801133
ActiveCN104059990AEasy to operateReduce use costMicrobiological testing/measurementGenotype AnalysisCoronary heart disease
The invention relates to a detection method and a kit for coronary heart disease susceptibility loci. The method comprises the following steps: (1) extracting a genome deoxyribonucleic acid (DNA) of a sample, and amplifying a region containing rs1801133 loci in a methylene tetrahydrofolate reductase (MTHFR) gene exon of the sample, so as to obtain an amplification product; (2) detecting the genetype of single nucleotide polymorphism (SNP) loci rs1801133 in the amplification product by using a high resolution melting (HRM) analysis technology. The genetype analysis is carried out on the region containing the rs1801133 loci in the amplified MTHFR gene by adopting the HRM analysis technology. The method is simple and quick to operate, low in use cost and accurate in result, and can achieve batch inspection; whether the tested people carry with coronary heart disease susceptibility genes can be clearly judged by using the kit, the coronary heart disease susceptibility people can be screened out from people, the poor living habit is improved, and the target of prevention is achieved.
Owner:光翰科技(上海)有限公司
Compositions and methods for the regulation of homocysteine levels within the body
InactiveUS7429569B2Reduce harmful metabolic waste productDecrease in levelBiocideSugar derivativesBetaineS-Adenosyl-l-methionine
Described herein is a method for reducing levels of the harmful metabolic waste product of S-adenosylmethionine (SAMe), homocysteine, and provide vitamin and other nutritional co-factors that reduce the production of homocysteine and either re-methylate homocysteine back to S-adenosylmethionine, or facilitate its conversion downstream to cystathione. The method of the invention may be achieved by administering 5-methyl tetrahydrofolate, methylcobalamin, and one or more compounds selected from the group consisting of betaine, pyridoxal-5-phosphate, N-acetyl-cysteine, and other cofactors.
Owner:FAST BALANCE
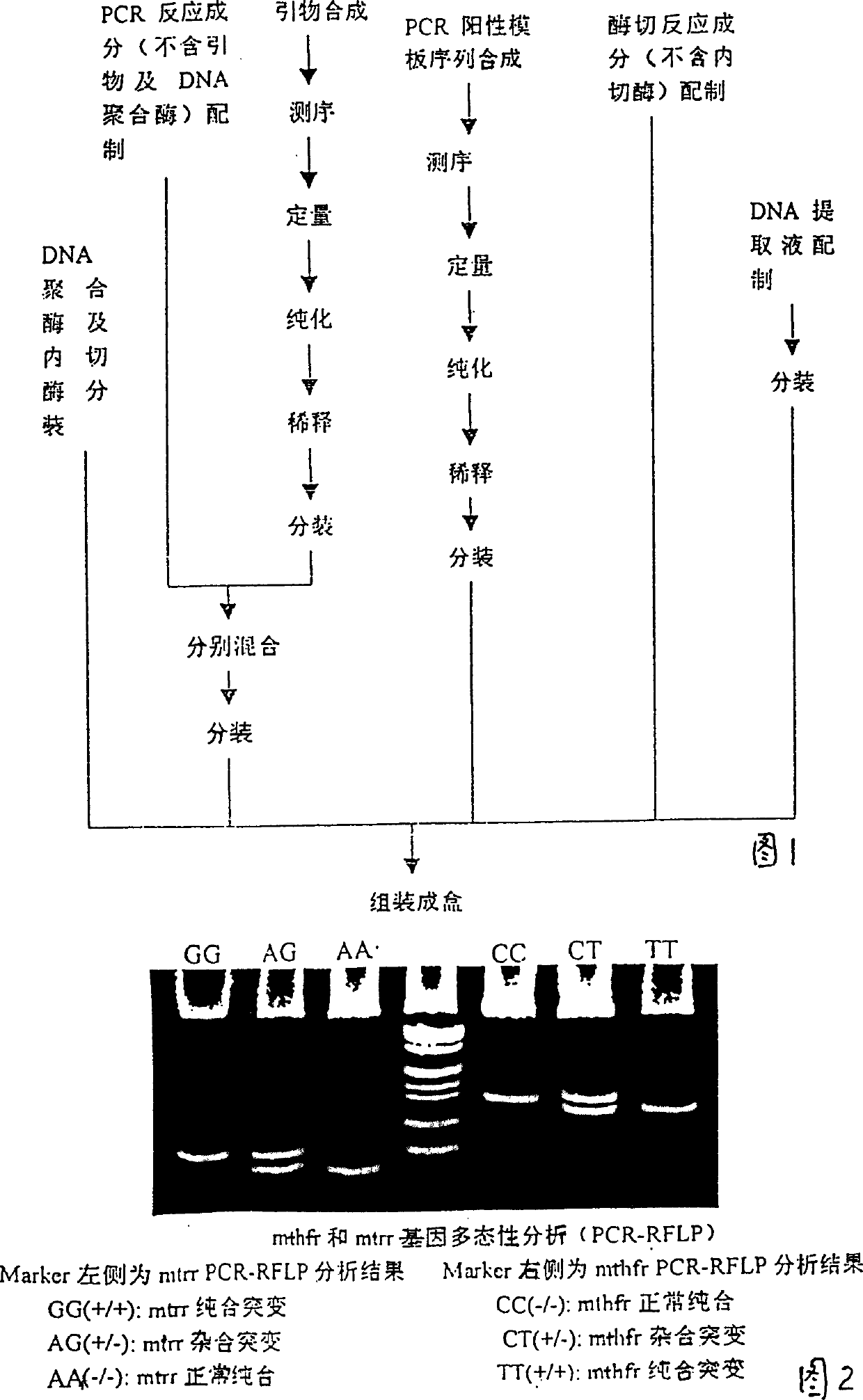
Pregestational gene warning diagnostic kit
InactiveCN1155722CGenetic polymorphism analysis method is matureSimple and fast operationMicrobiological testing/measurementPositive controlGenomic DNA
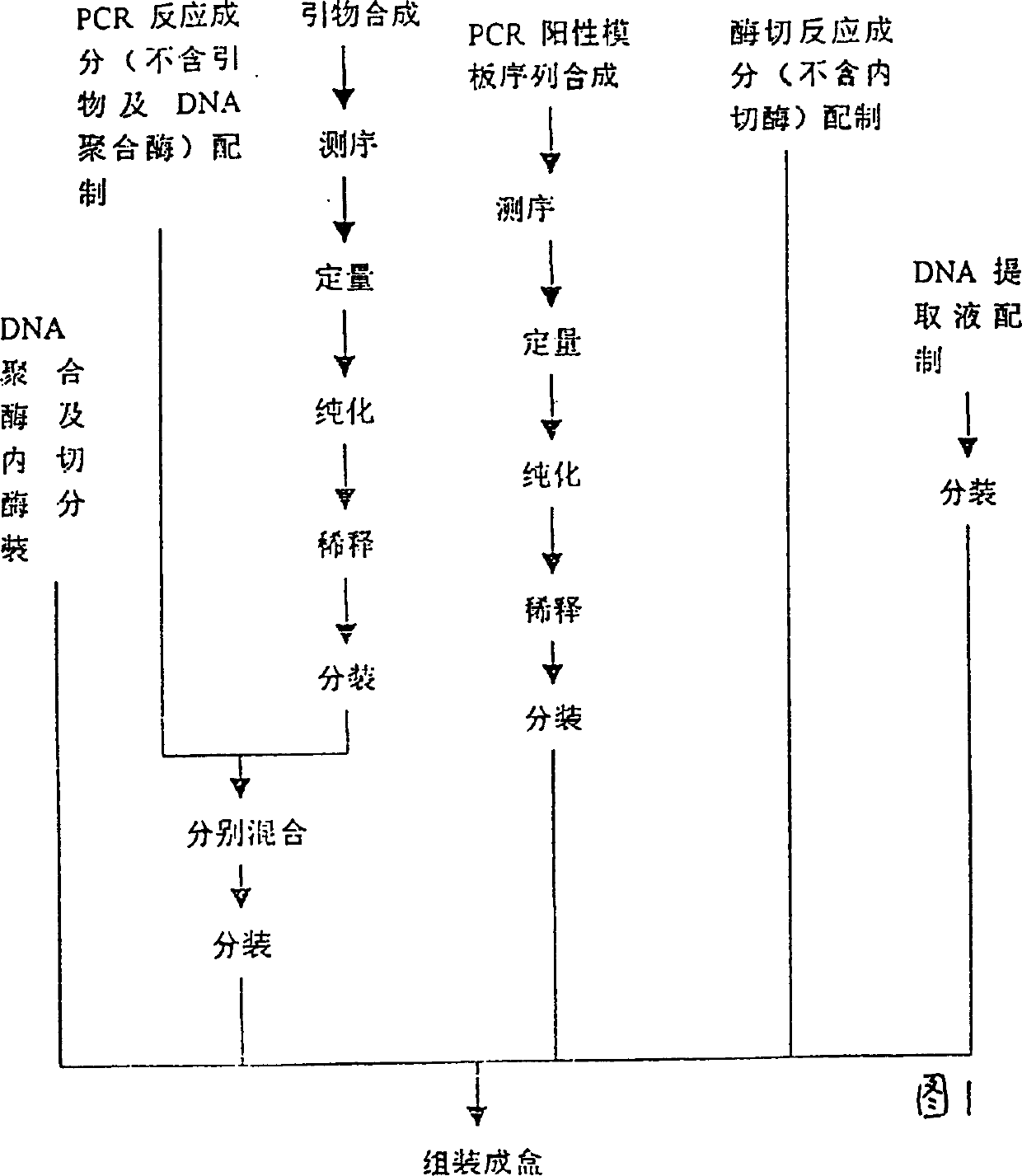
The present invention relates to a pregestational gene alarm diagnosis kit and its detection method, including DNA extraction liquor for extracting DNA template of testee, PCR mixed liquor for amplifying specific fragment on the genomic DNA of testee, DNA polymerase, PCR product enzyme excision mixed liquor, restriction endonuclease, positive control template of homozygous mutation and coupling pipe for making PCR reaction. The described alarm gene includes N5, N10-methylene tetrahydrofolate reductase and methionine synthetase reductase gene, their mulant sites are respectively 667-cytosine-thymine and 66 adenine-guanine, and their primer sequences respectively are 5'-TGA AGG AGA AGG TGT CTG CGG GA-3', 5'-AGG ACG GTG CGG TGA GAG TG-3', 5'-GCA AAG GCC ATC GCA GAA GAC AT-3' and 5'-GTG AAG ATC TGC AGA AAA TCC ATG TA-3'. It can be used for pregestational diagnosis of that filial generation produces neural tube deform or not.
Owner:SICHUAN UCAN BIO TECH
Stable pharmaceutical composition containing folates
ActiveUS9642853B2Organic active ingredientsPharmaceutical delivery mechanismSolubilityFolic acid antagonist
The administration of leucovorin as well as other active, reduced folates is useful as an antidote to drugs which act as folic acid antagonists and in combination chemotherapy with 5-FU. The most often used calcium salts of the folates have a low solubility in water and form almost insoluble degradation products. Therefore, aqueous solutions are unstable and precipitates result. Precipitates in injectable products present an unacceptable safety risk to patients. Stable high strength pharmaceutical aqueous compositions are formed containing calcium salts, magnesium or zinc salts of the reduced folates leucovorin, (6R,S)-tetrahydrofolic acid, (6S)-tetrahydrofolic acid, 5,10-methylene-(6R,S)-tetrahydrofolate, 5,10-methylene-(6R)-tetrahydrofolate, 5-methyl-(6R,S)-tetrahydrofolate or 5-methyl-(6S)-tetrahydrofolate and one or more of the compounds sodium gluconate, potassium gluconate, glycerophosphate disodium salt or glycerophosphate dipotassium salt.
Owner:APROFOL
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com