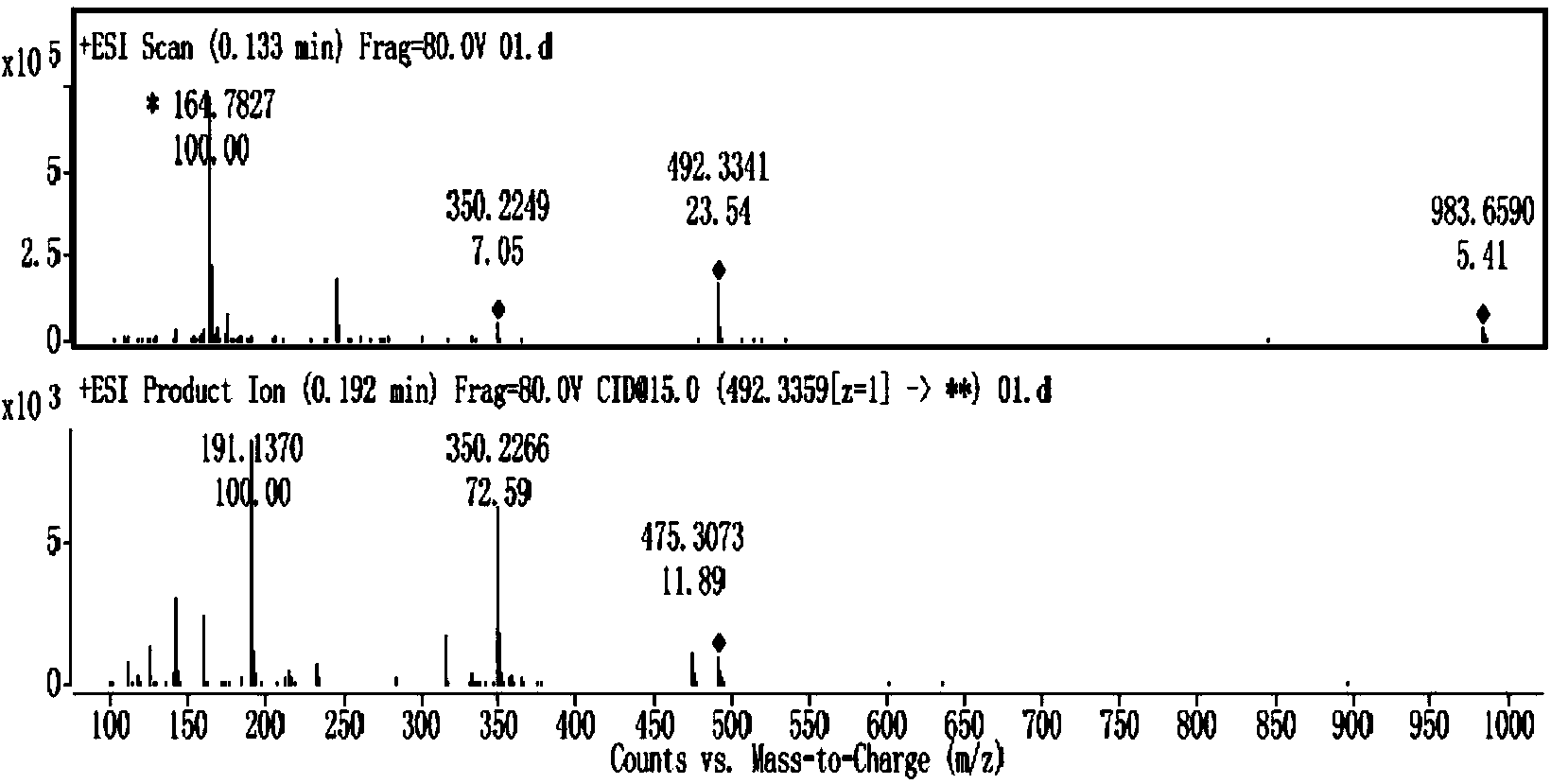
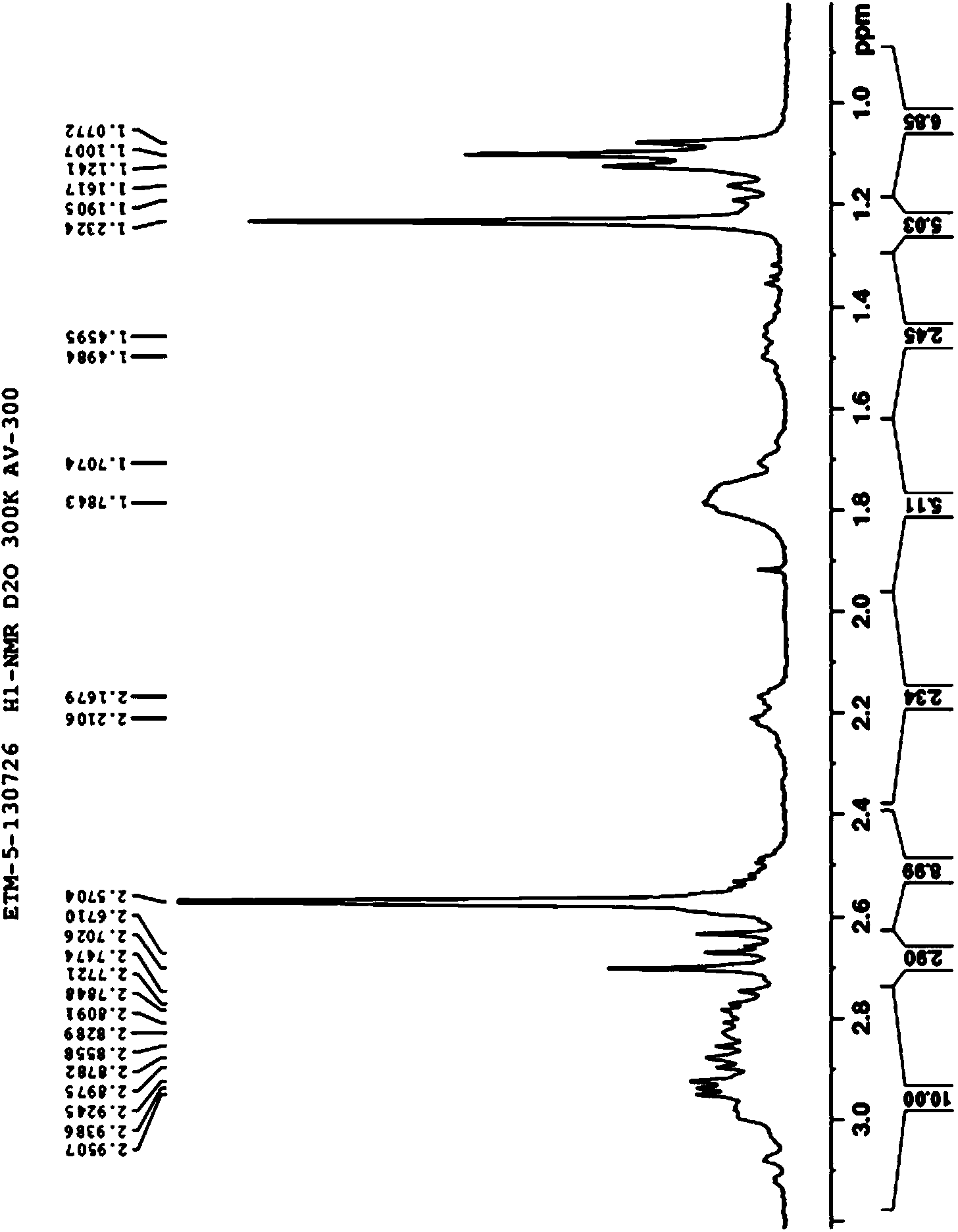
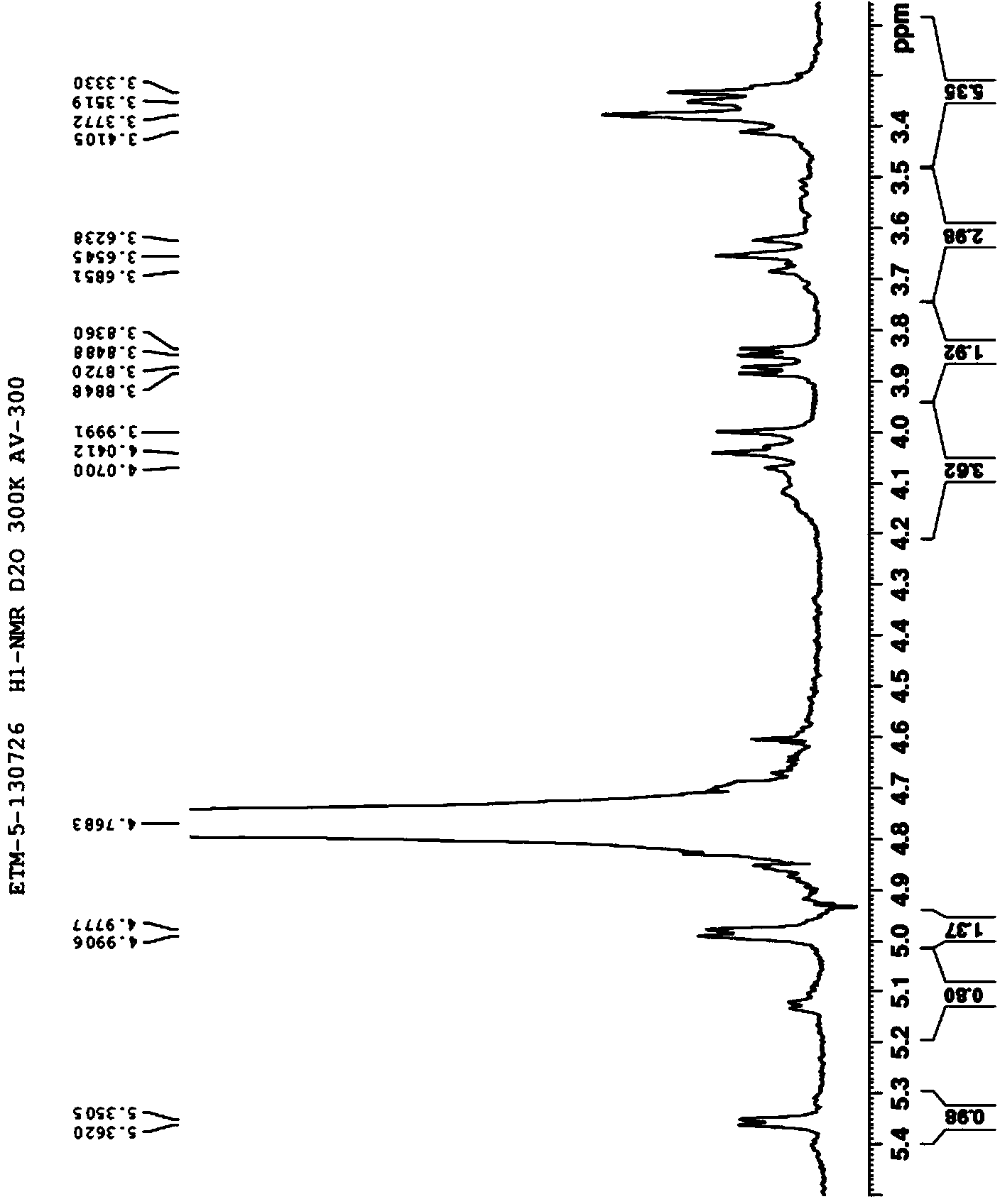
Aminoglycoside compound and extraction separation method thereof
A compound and composition technology, applied in the field of new aminoglycoside compounds and their extraction and separation, can solve the problems of synthesis errors, affecting the whole process of bacterial protein synthesis, hindering the synthesis of initial complexes, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Get 20 grams of the etimicin crude product produced by the prior art, add 40ml purified water to dissolve, the aqueous solution flows down slowly along the column inner wall, load the sample on the HD-2 weakly acidic cation exchange resin column, and first elute with purified water, Then use gradient elution with concentrations of 1%, 1.5%, 2%, and 3% ammonia water, TLC detection (developing solvent: chloroform-methanol-ammonia water, 1:1:1, take the lower layer), combine the fractions to obtain Fractions enriched in impurities. Concentrate the impurity-enriched fractions respectively, freeze-dry to obtain 10 grams of solid mixture, take 300 grams of 100-200 mesh silica gel, and separate by silica gel column chromatography (chloroform:methanol:ammonia water (3:1:1, take the lower layer ) and preparative liquid phase purification (with 0.2mol / L trifluoroacetic acid aqueous solution:methanol=84:16 as the mobile phase), the fraction containing the target compound was conce...
Embodiment 2
[0056] Embodiment 2, the preparation of hydrochloride:
[0057] Get 20 grams of the etimicin crude product produced by the prior art, add 40ml purified water to dissolve, the aqueous solution flows down slowly along the column inner wall, load the sample on the HD-2 weakly acidic cation exchange resin column, and first elute with purified water, Then use gradient elution with concentrations of 1%, 1.5%, 2%, and 3% ammonia water, TLC detection (developing solvent: chloroform-methanol-ammonia water, 1:1:1, take the lower layer), combine the fractions to obtain Fractions enriched in impurities. Concentrate the impurity-enriched fractions respectively, freeze-dry to obtain 10 grams of solid mixture, take 300 grams of 100-200 mesh silica gel, and separate by silica gel column chromatography (chloroform:methanol:ammonia water (3:1:1, take the lower layer ) and preparative liquid phase purification (with 0.2mol / L trifluoroacetic acid aqueous solution:methanol=84:16 as the mobile pha...
Embodiment 3
[0058] Embodiment 3, the preparation of sulfate:
[0059] Get 20 grams of the etimicin crude product produced by the prior art, add 40ml purified water to dissolve, the aqueous solution flows down slowly along the column inner wall, load the sample on the HD-2 weakly acidic cation exchange resin column, and first elute with purified water, Then use gradient elution with concentrations of 1%, 1.5%, 2%, and 3% ammonia water, TLC detection (developing solvent: chloroform-methanol-ammonia water, 1:1:1, take the lower layer), combine the fractions to obtain Fractions enriched in impurities. Concentrate the impurity-enriched fractions respectively, freeze-dry to obtain 10 grams of solid mixture, take 300 grams of 100-200 mesh silica gel, and separate by silica gel column chromatography (chloroform:methanol:ammonia water (3:1:1, take the lower layer ) and preparative liquid phase purification (with 0.2mol / L trifluoroacetic acid aqueous solution:methanol=84:16 as the mobile phase), t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com