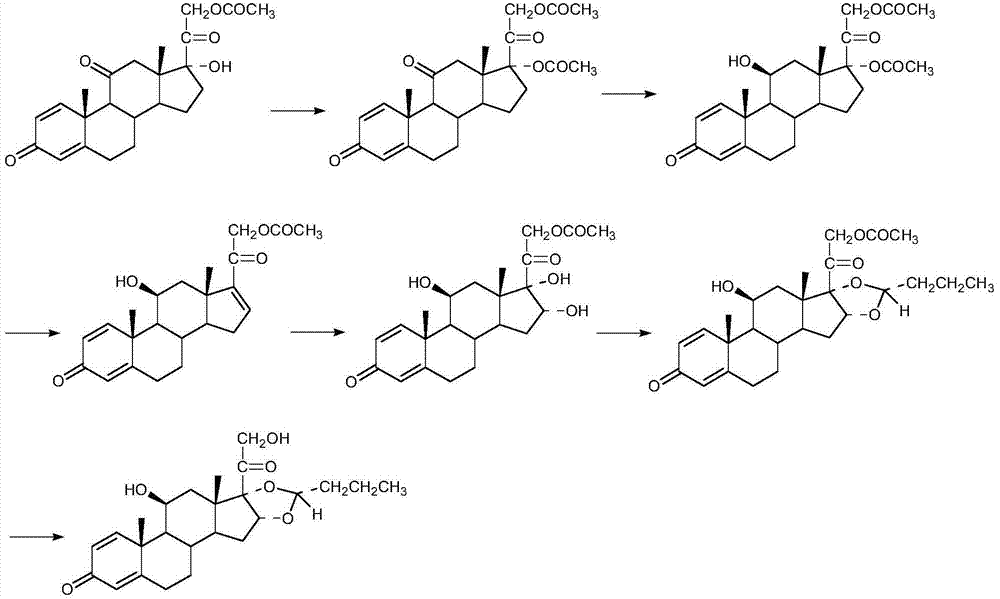
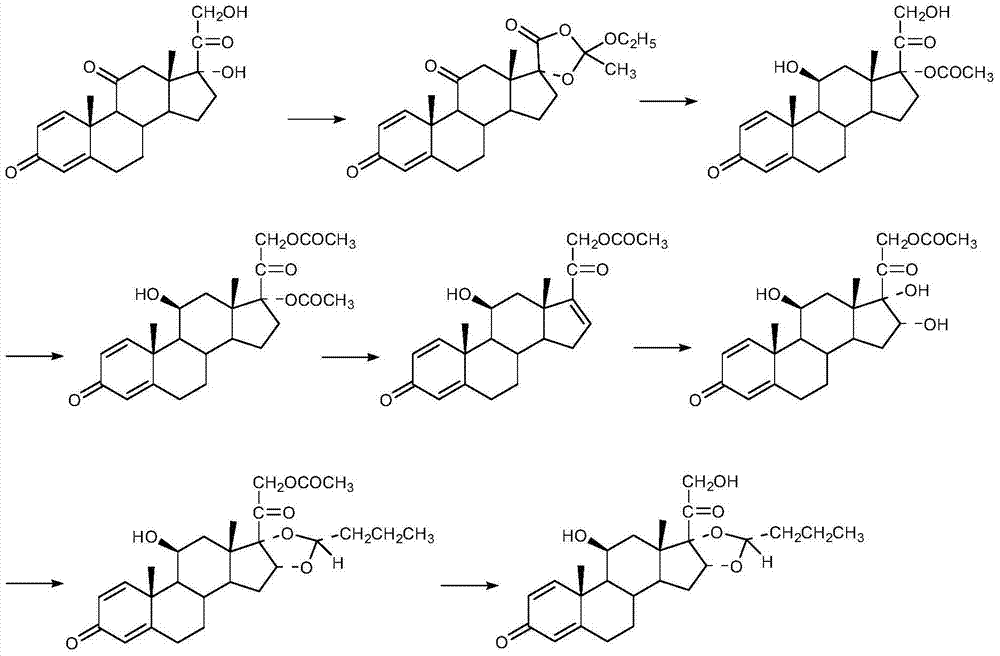
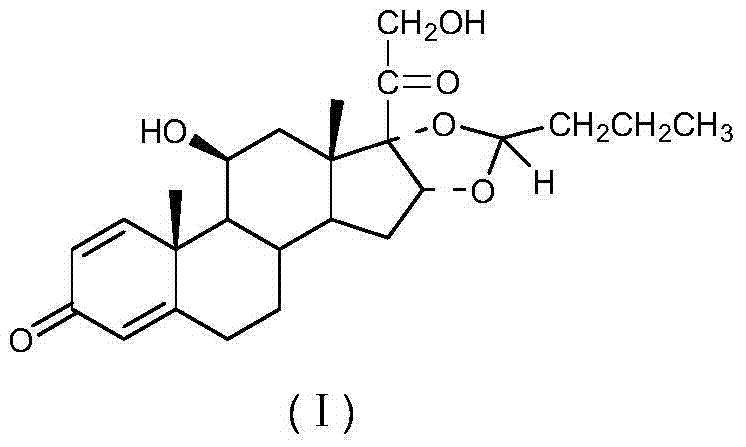
Preparation method of (R)-budesonide
A single technology of dexbudesonide, which is applied in the field of preparation of dexbudesonide, can solve the problems of high toxicity and high price of intermediate reagents, and achieve the effect of high yield and good separation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Put 11β,21-dihydroxy-16α,17-[(1-methylethylene)-dioxy]pregna-1,4-diene-3,20-dione (desonide ) 5kg, purified water 80kg, ethanol 80kg, reflux to dissolve completely. The temperature was lowered to 25°C, and 0.8 kg of acetone was added dropwise, and the reaction was kept for 5 hours after the drop was completed. Concentrate in vacuo at 50°C and centrifuge. The solid was dissolved with 100 kg of ethanol, 2.2 kg of (+)-tartaric acid was added, and the temperature was raised to 65° C. for 2 hours. After the reaction was completed, the temperature was lowered to 5° C., and centrifuged to obtain dexbudesonide tartrate.
[0027] R isomer: 99.2%; S isomer: 0.8%
[0028] [a]D 20° =+116.4°(C=1,CH 2 Cl 2 )
[0029] 1 H-NMR (DCCl 3 , 400MHz): 7.28(t,1H), 6.26(d,1H), 6.02(S,1H), 5.15~5.19(m,1H), 4.48~4.91(m,3H), 4.23(t,1H), 3.01(S, 1H), 2.56(t, 1H), 2.34(d, 1H), 2.07~2.18(m, 3H), 1.47~1.51(m, 1H), 1.46(S, 3H), 1.32~1.43( m, 2H), 1.10 ~ 1.18 (m, 2H), 0.89 ~ 0.99 (m, 6H)
...
Embodiment 2
[0032] Put 3kg of dexbudesonide tartrate in the above example into the reactor, add 10kg of purified water and 10kg of ethanol. Cool down to 0°C, add dropwise 0.5% sodium bicarbonate solution to adjust the pH to 7.5. Concentrate in vacuo to stop flow, centrifuge, and wash with purified water to obtain 1.8 kg of dexbudesonide.
[0033] R isomer: 99.2%; S isomer: 0.8%
Embodiment 3
[0035] Put 11β,21-dihydroxy-16α,17-[(1-methylethylene)-dioxy]pregna-1,4-diene-3,20-dione (desonide ) 5kg, purified water 80kg, ethanol 80kg, reflux to dissolve completely. The temperature was lowered to 25°C, and 0.8 kg of acetone was added dropwise, and the reaction was kept for 5 hours after the drop was completed. Concentrate in vacuo and centrifuge. The solid matter was put into a reaction kettle, 100 kg of ethanol and 4 kg of (+)-camphorsulfonic acid were added, and the temperature was raised to 65° C. for 2 hours. After the reaction was completed, the temperature was lowered to 5° C., and centrifuged to obtain dexbudesonide tartrate.
[0036] R isomer: 99.7%; S isomer: 0.3%
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com