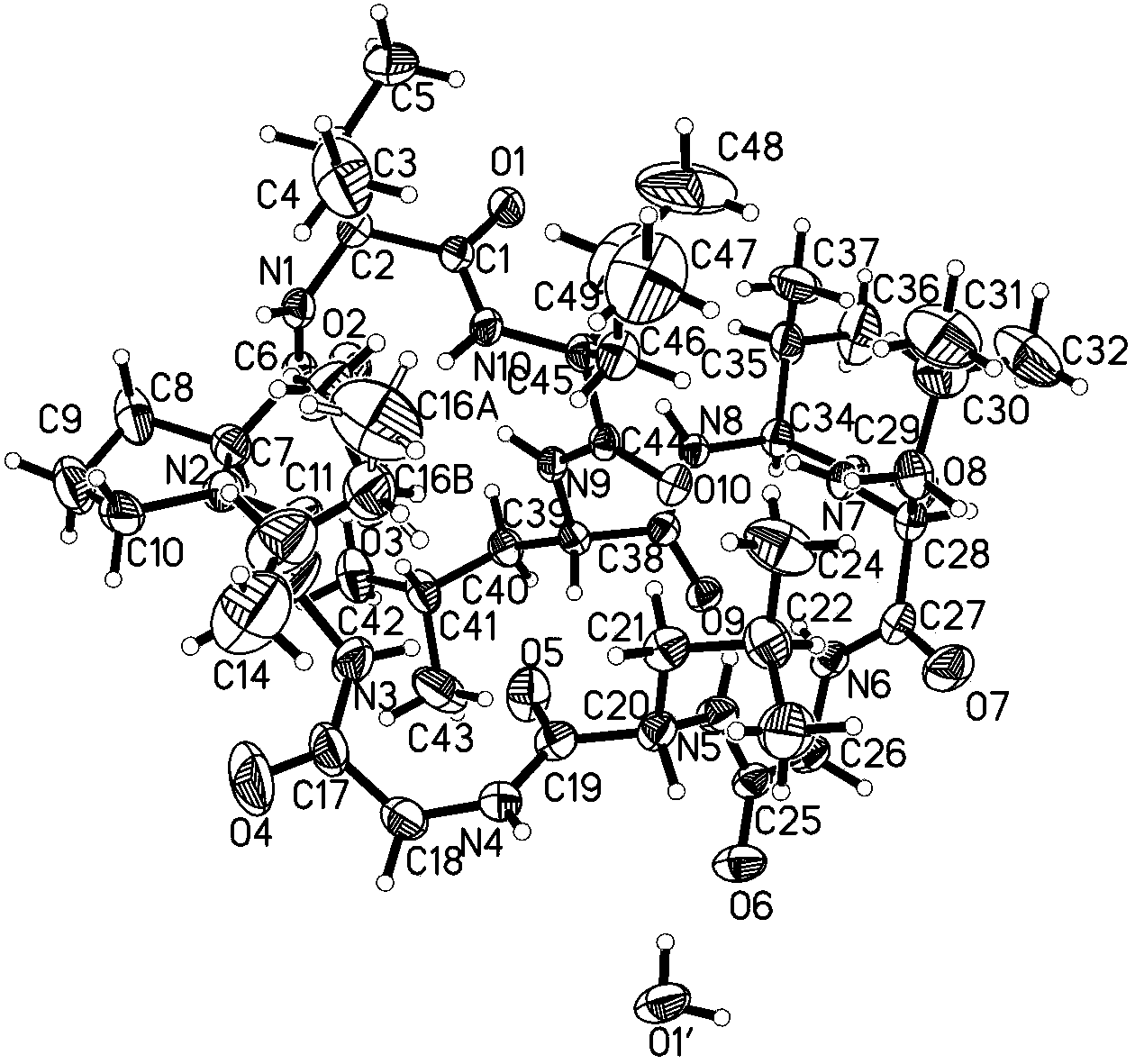
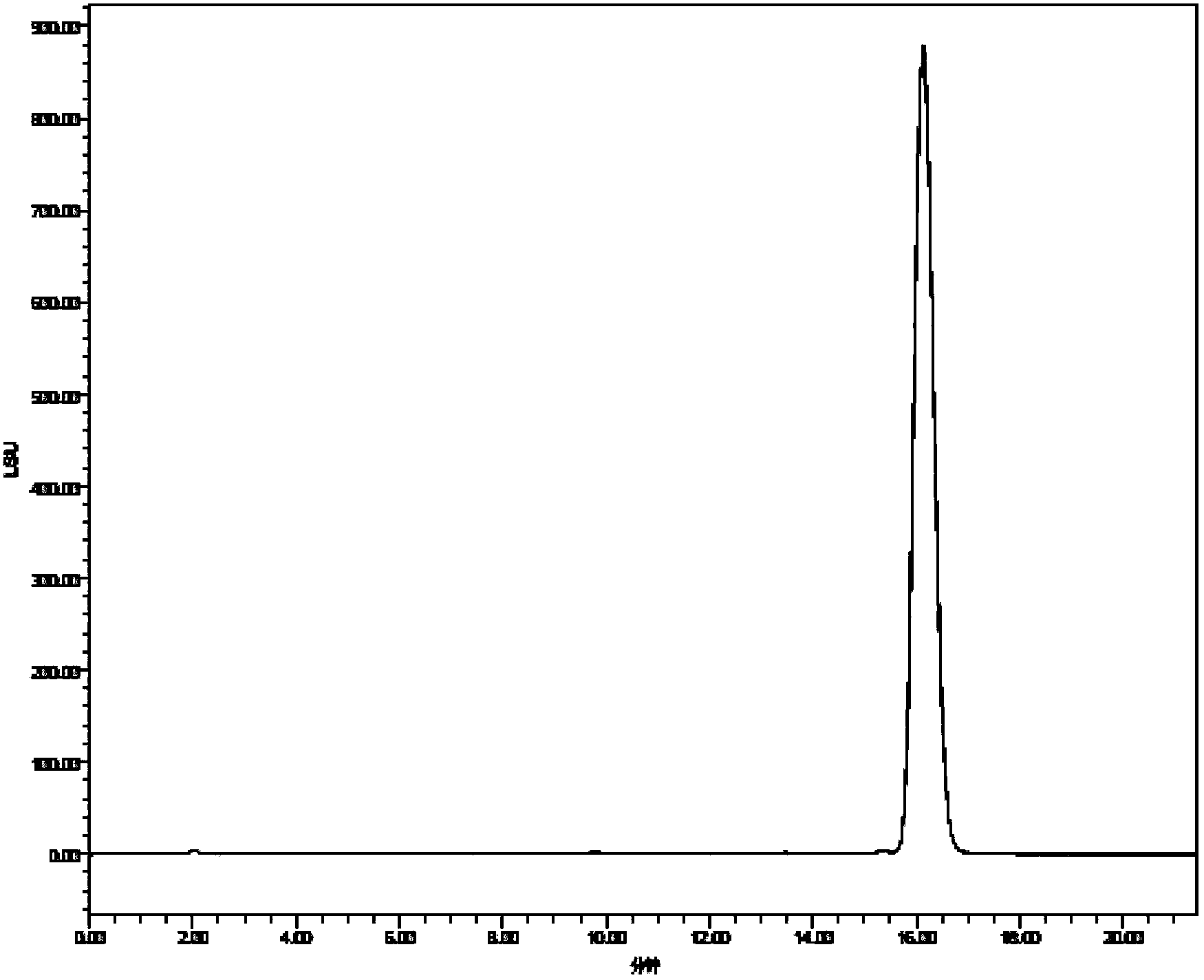
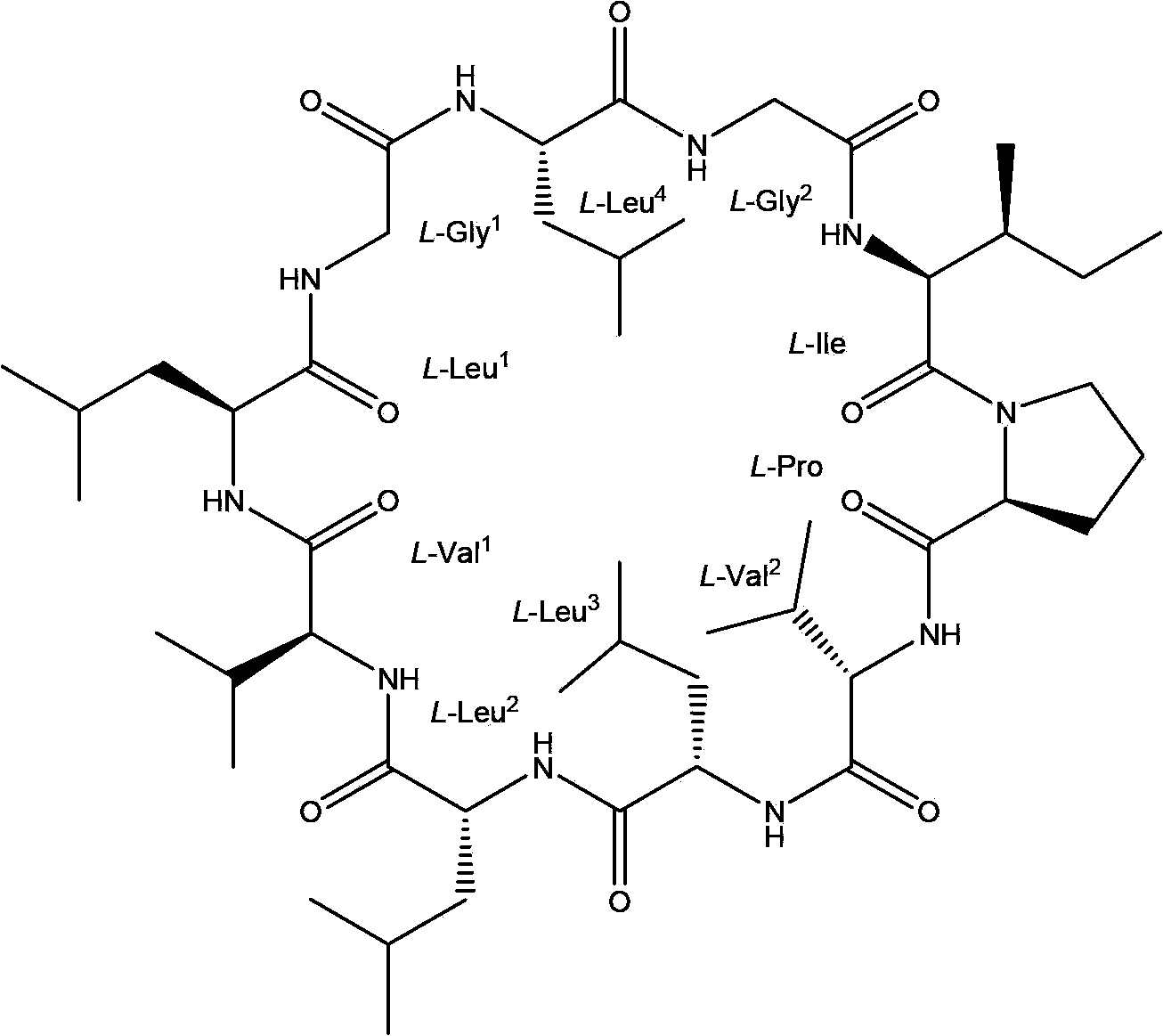
Chemical preparation method of cyclic decapeptide compound GG-110824
A technique for compound GG-110824, which is applied in the field of chemical preparation of cyclic decapeptide compound GG-110824, can solve the problems of difficult synthesis, low yield, and long distance, and achieve simple preparation steps, high practical value, and high yield high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1 chemical preparation GG-110824
[0027] The chemical preparation methods include solid-phase synthesis of linear peptides, liquid-phase ring closure, solid-phase column chromatography separation and recrystallization;
[0028] 1. Synthesis of GG-110824 linear precursor
[0029] (1) Take the resin: take a certain amount of Fmoc-Gly-Wang resin, and swell it in DMF for two hours;
[0030] (2) Removal of Fmoc protecting group: Stir and react with nitrogen in 20% piperidine / DMF solution for 30 minutes; then wash with equal volume of DMF repeatedly for 5 times, each time for 3 minutes;
[0031] (3) Ninhydrin detection: Take a small amount of the reacted resin in a test tube, add 2 drops of 1% (volume fraction, the same below) ninhydrin / ethanol solution, heat for 2-3 minutes; the resin is blue-purple or purple-red , it means that the deprotection is successful, proceed to the next step of the reaction; if the resin does not change color, it means that the deprotec...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com