Method for synthesizing 2,2,4,4-tetramethyl-1,3-cyclobutanediol
A cyclobutanediol and synthesis method technology, applied in 2 fields, can solve the problems of high cost of raw materials, harsh requirements on reaction vessel and operating conditions, and low yield of cyclocondensation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
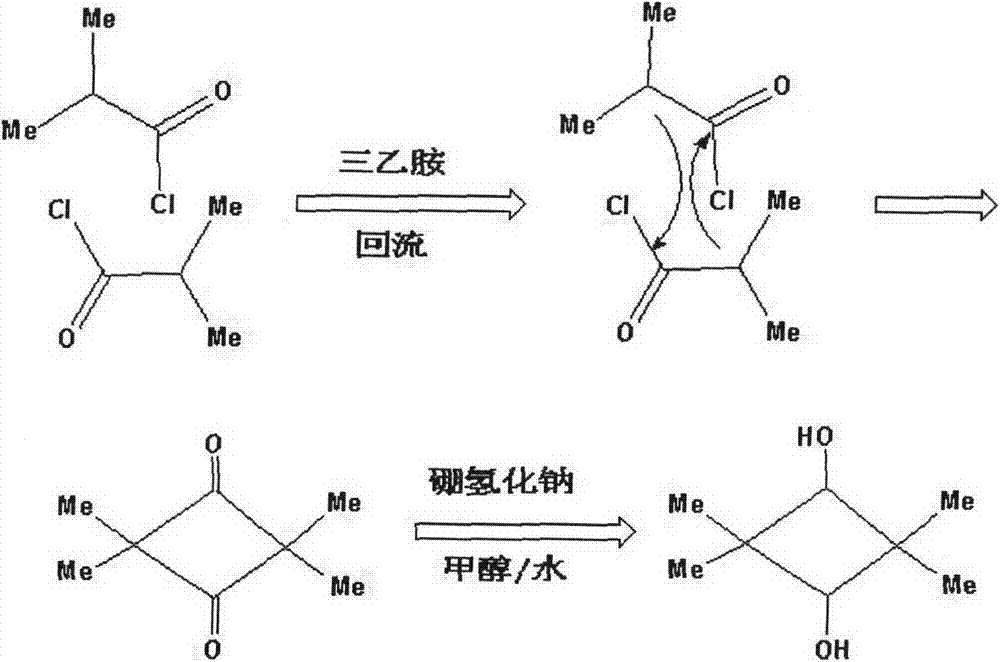
[0015] In a three-necked flask equipped with a condenser, 5.3g (0.05mol) isobutyryl chloride and 40mL ether were added, and 8.6g (0.085mol) triethylamine was added under stirring evenly. The system was heated to reflux and reacted for 15h, and then the system was cooled. Wash with dilute hydrochloric acid until the white turbidity is dissolved and separate. The aqueous layer was extracted with ether, the organic phases were combined, and part of the solvent was evaporated under reduced pressure to obtain a mixed solution of a white intermediate.
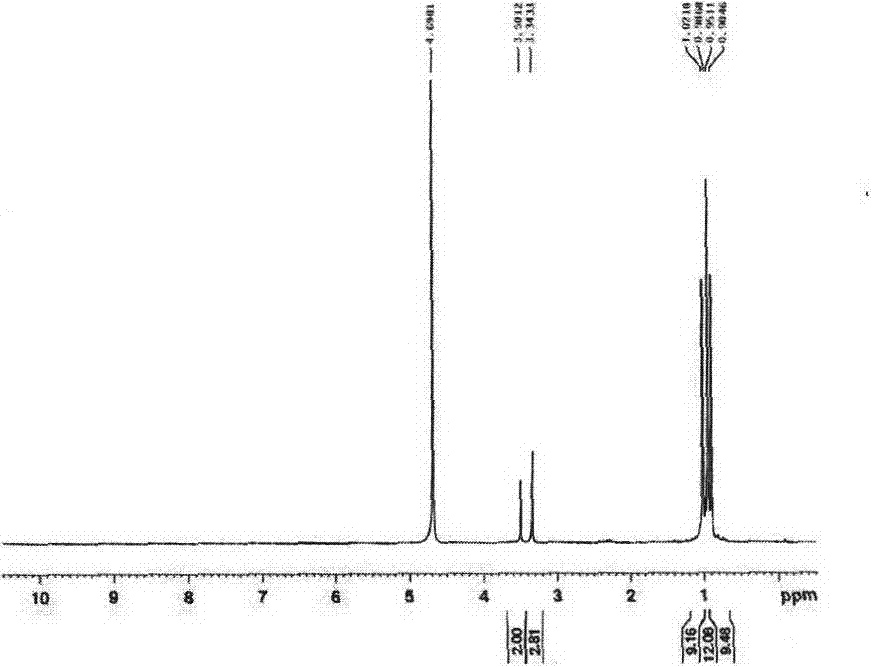
[0016] Add the above-mentioned mixture containing intermediates to a three-necked flask, then add it to a 50mL methanol and 50mL water system, adjust the pH of the solution to alkaline with 10% NaOH, and add 2g (0.053mol) in batches under ice bath conditions NaBH 4 After the addition, react for 4h at room temperature, quench with dilute hydrochloric acid, spin-dry the reaction solution, and extract with 100mL absolute ethanol. The extra...
Embodiment 2
[0018] In a three-necked flask equipped with a condenser, 5.3g (0.05mol) isobutyryl chloride and 40mL propyl ether were added, and 8.6g (0.085mol) triethylamine was added under stirring evenly. The system was heated to reflux and reacted for 15h, and then the system was cooled. Wash with dilute hydrochloric acid until the white turbidity is dissolved and separate. The aqueous layer was extracted with propyl ether, the organic phases were combined, and part of the organic solvent was evaporated under reduced pressure to obtain a mixed solution in which a white intermediate was precipitated.
[0019] Add the above-mentioned mixed solution containing intermediates into a three-necked flask, then add it to 70mL methanol and 30mL water system, adjust the pH of the solution to alkaline with 10% NaOH, add 2g (0.053mol) in batches under ice bath conditions NaBH 4 After the addition, react for 4h at room temperature, quench with dilute hydrochloric acid, spin-dry the reaction solution, an...
Embodiment 3
[0021] Add 5.3g (0.05mol) isobutyryl chloride and 40mL ethylene glycol diethyl ether into a three-necked flask equipped with a condenser, add 8.6g (0.085mol) triethylamine under stirring evenly, and then heat and reflux for 15h to cool the system. Wash with dilute hydrochloric acid until the white turbidity dissolves, separate the layers, extract the aqueous layer with ethylene glycol diethyl ether, combine the organic phases, and evaporate part of the solvent under reduced pressure to obtain a mixture of precipitated white intermediates.
[0022] Add the above-mentioned mixture containing intermediates to a three-necked flask, and then add it to the 30mL methanol and 70mL water system, adjust the pH of the solution to alkaline with 10% NaOH, add 2g (0.053mol) in batches under ice bath conditions NaBH 4 After the addition, react for 4h at room temperature, quench with dilute hydrochloric acid, spin-dry the reaction solution, and extract with 100mL absolute ethanol. The extract is ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com