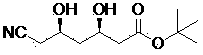
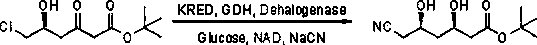Biological preparation method of (3R, 5R)-6-cyano-3,5-dyhydroxytert-butylhexanoate
A technology of tert-butyl hydroxycaproate and tert-butyl hexanoate, applied in the field of biopharmaceuticals, can solve the problems of complicated operation steps and high cost, and achieve the effects of simplifying operation steps, reducing production process and low raw material price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment 1: the composite enzyme dry powder shake flask production process of recombinant ketoreductase and glucose dehydrogenase
[0026] Inoculate a single colony of Escherichia coli carrying a ketoreductase gene and a glucose dehydrogenase gene from a glycerol tube or transformation plate into 10ml of liquid LB medium containing double resistance to ampicillin and kalamycin, at 37°C Activation on a shaking table for 12 hours (150rpm), inoculate the above culture into 100ml liquid LB medium containing double resistance to ampicillin and kalamycin at 1 / 100 inoculum amount, and expand the culture on a shaking table at 37°C ( 150rpm), to OD 600When the value reaches 1, add isopropyl-β-D-thiogalactoside, continue to incubate at 37°C for 6 hours, centrifuge, collect the precipitate, add 10ml of triethanolamine hydrochloride buffer (0.1M, pH=7.0) Suspension, put the suspension in an ice-water bath for 15 minutes to ultrasonically break the wall, then centrifuge, pre-free...
Embodiment 2
[0027] Embodiment 2: production process of dehalogenase dry powder shake flask
[0028] Inoculate 10 ml of ampicillin-containing liquid LB medium from a glycerol tube or a single colony on a transformation plate (a single colony of recombinant Escherichia coli carrying a dehalogenase gene), and activate it on a shaker at 37°C for 12 hours (150rpm). The culture was inoculated into 100ml liquid LB medium containing ampicillin at 1 / 100 inoculum, and expanded on a shaking table (150rpm) at 37°C until OD 600 When the value reaches 1, add isopropyl-β-D-thiogalactoside, continue to incubate at 37°C for 6 hours, centrifuge, collect the precipitate, add 10ml of triethanolamine hydrochloride buffer (0.1M, pH=7.0) Suspension, the suspension is placed in an ice-water bath for 15 minutes to ultrasonically break the wall, and then centrifuged, the supernatant is pre-frozen until the temperature drops to -20°C, and then freeze-dried for 24 hours to obtain a dry powdered dehalogenase.
Embodiment 3
[0030] In the reaction vessel, 50 ml of 0.1M triethanolamine hydrochloride buffer solution with a pH of 7 was first added, followed by adding 0.15 g of recombinant ketoreductase and glucose dehydrogenase compound enzyme (prepared in Example 1), and dehalogenase (prepared in Example 2) ) 0.15g, 10mg NAD, 15g glucose and 10g (5R)-6-chloro-5-hydroxy-3-oxohexanoic acid tert-butyl ester, stir well, and maintain pH6.8~7.2 with 30% NaCN aqueous solution , stirred at room temperature for 12 hours and the reaction was completed. Ethyl acetate was added for multiple extractions. The organic phases were combined and the solvent was evaporated under reduced pressure to obtain 8.91 g of (3R,5R)-6-cyano-3,5-dihydroxyhexyl Acid tert-butyl ester, a light yellow oily liquid.
[0031] product structure 1 H NMR, 13 C NMR and ESI-HRMS detection, the results are as follows: 1 H NMR (400MHz, CDCl 3 ):δ=4.25(bs, 1H), 4.34-4.23(m, 1H), 4.21(bt, 1H), 3.96(bd, 1H), 2.55(dd, J = 5.5, 11.1 Hz, 2H),...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com