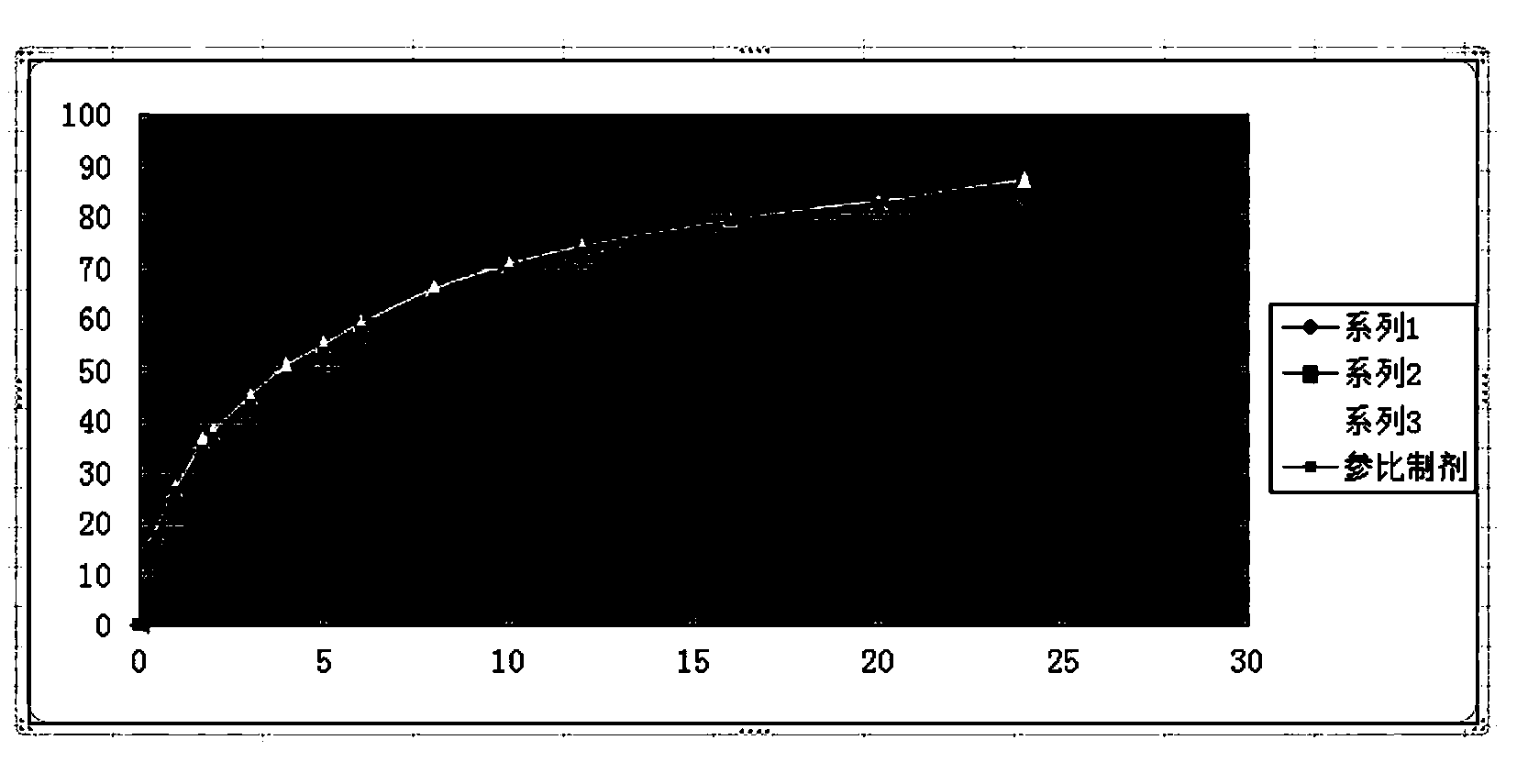
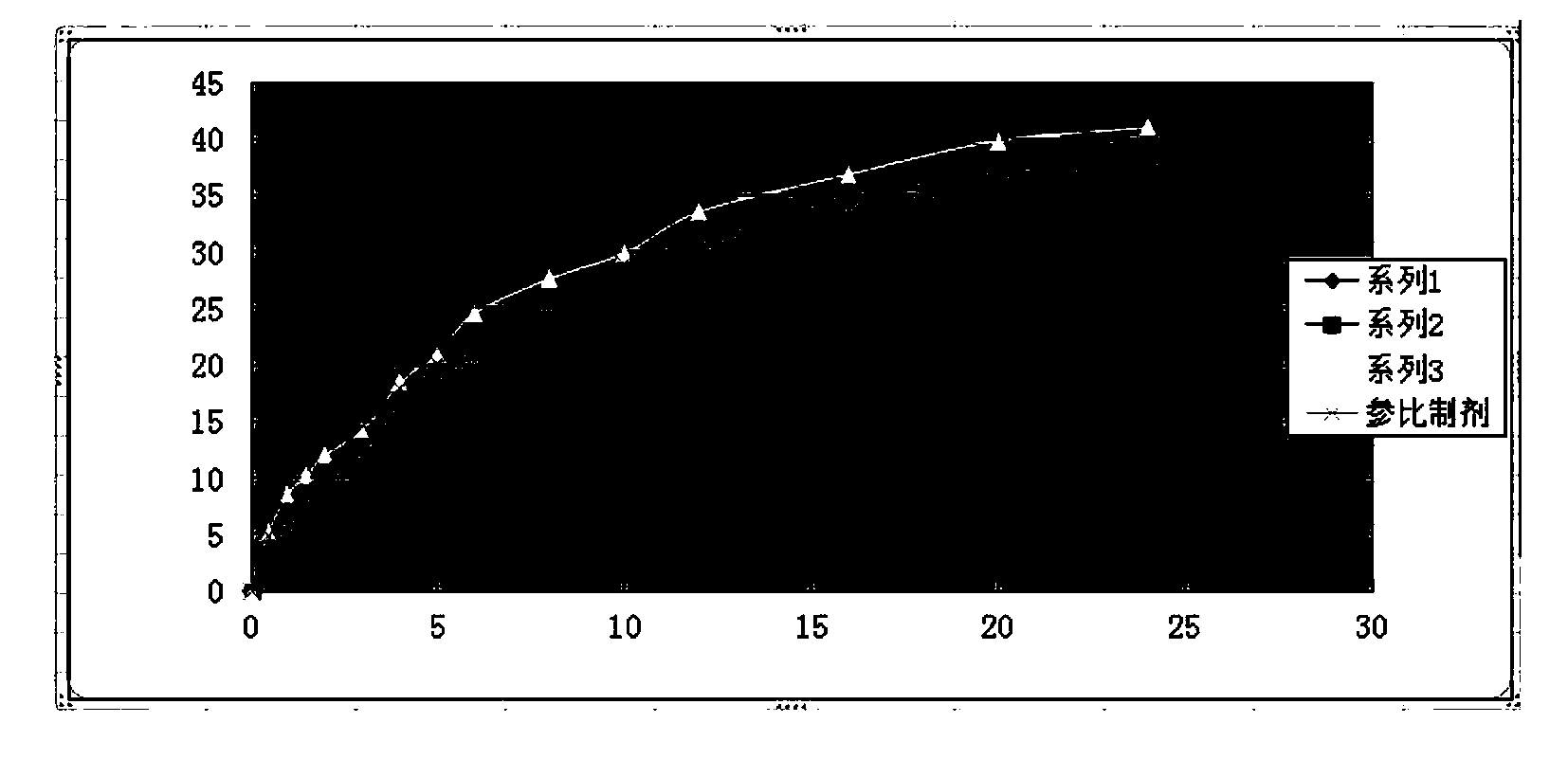
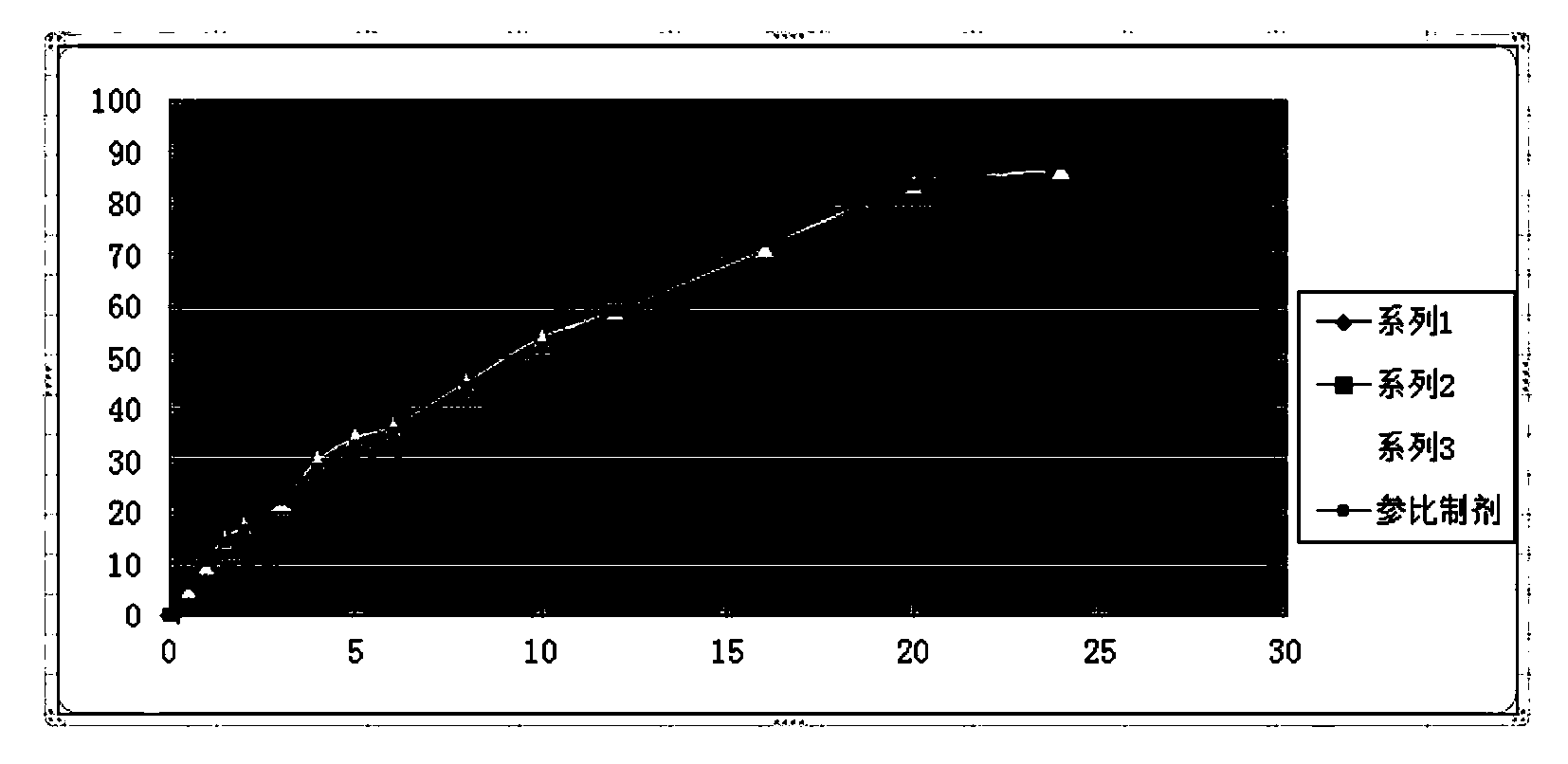
Guanfacine hydrochloride sustained-release preparation and preparation method thereof
A technology for guanfacine hydrochloride and sustained-release preparations, which is applied in pharmaceutical formulations, medical preparations containing active ingredients, and drug delivery, and can solve the problem of unsatisfactory pharmacokinetic parameters such as bioavailability in vivo, large batch-to-batch differences, and Poor release stability and other issues, to achieve good stability and dissolution characteristics, reduce the production of impurities, and reduce the effect of production energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Based on the specification of 1 mg free guanfacine effective content in each sustained-release preparation, the raw material composition of each guanfacine hydrochloride sustained-release tablet is: guanfacine hydrochloride 1.14 mg, pregelatinized starch 41.6 mg, hydroxymethyl cellulose Sodium Sulfate 46mg, Hydroxyethylcellulose 36mg, Citric Acid 17mg, Magnesium Lauryl Sulfate 4mg and Croscarmellose Sodium 4.26mg.
[0035] In this embodiment, according to the above-mentioned specifications of 1 mg free guanfacine hydrochloride effective content in each sustained-release preparation, the raw materials for producing 5000 guanfacine hydrochloride sustained-release tablets consist of: 5.7 g of guanfacine hydrochloride, 208 g of pregelatinized starch, Hydroxymethylcellulose sodium 230g, hydroxyethylcellulose 180g, citric acid 85g, magnesium lauryl sulfate 20g and croscarmellose sodium 21.3g.
[0036] In addition, according to the raw material composition of 5000 guanfacine h...
Embodiment 2
[0038] Based on the specification of free guanfacine effective content of 1 mg in each sustained-release preparation, the raw material composition of each guanfacine hydrochloride sustained-release tablet is: guanfacine hydrochloride 1.14 mg, pregelatinized starch 40 mg, hydroxymethylcellulose Sodium 49.4mg, hydroxyethylcellulose 30mg, tartaric acid 20.6mg, polyethylene glycol 4000 4.4mg and cross-linked polyvinylpyrrolidone 4.26mg.
[0039] In this embodiment, according to the specification of free guanfacine effective content of 1 mg in each sustained-release preparation mentioned above, the raw materials for producing 5000 guanfacine hydrochloride sustained-release tablets consist of: 5.7 g of guanfacine hydrochloride, 200 g of pregelatinized starch, Hydroxymethylcellulose sodium 247g, hydroxyethylcellulose 150g, tartaric acid 103g, polyethylene glycol 4000 22g and cross-linked polyvinylpyrrolidone 21.3g.
[0040] In addition, according to the raw material composition of 50...
Embodiment 3
[0042] Based on the specification of 1 mg free guanfacine effective content in each sustained-release preparation, the raw materials of each guanfacine hydrochloride sustained-release tablet are: guanfacine hydrochloride 1.14 mg, pregelatinized starch 57 mg, Eudragit 100 80.8 mg, malic acid 22.86mg, polyethylene glycol 4000 5.4mg and croscarmellose sodium 6mg.
[0043] In this embodiment, according to the specification of 1 mg free guanfacine effective content in each sustained-release preparation mentioned above, the raw materials for producing 5000 guanfacine hydrochloride sustained-release tablets are composed of: 5.7 g of guanfacine hydrochloride, 285 g of pregelatinized starch, Eudragit 100 404g, malic acid 114.3g, polyethylene glycol 4000 27g and croscarmellose sodium 30g.
[0044] In addition, according to the raw material composition of 5000 guanfacine hydrochloride sustained-release tablets, 2500 guanfacine hydrochloride sustained-release tablets with an effective fre...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com