Method for gene transfection by utilizing PEG (polyethylene glycol) functionalized PAMAM (poly(amidoamine)) dendrimer carrier encapsulating gold nanoparticles
A technology of nano-gold particles and dendrimers, applied in the field of gene transfection of PAMAM dendrimers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
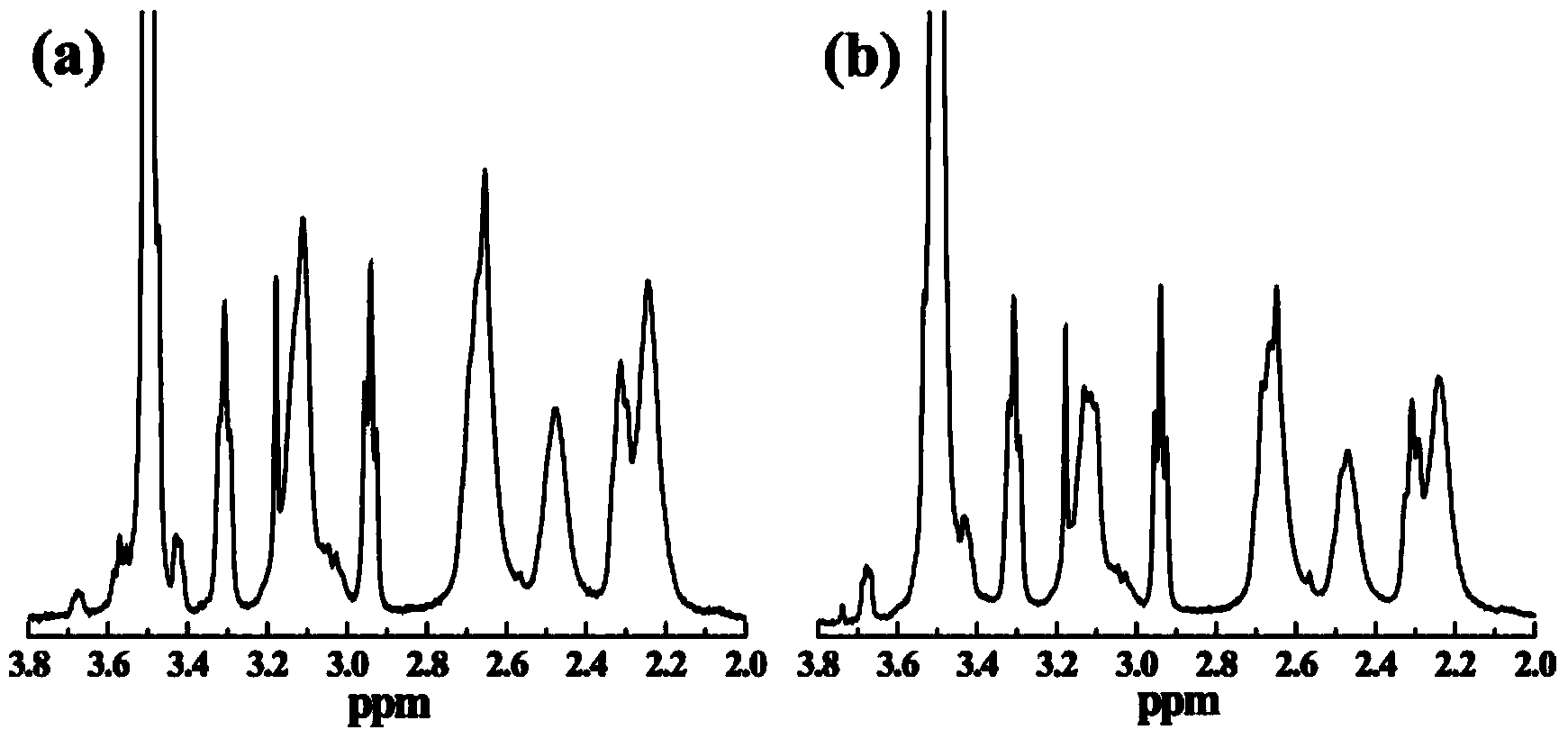
[0064]Taking the combination of carrier H1 as an example, weigh 10 mg of (mPEG-COOH) with a molecular weight of 2000 and dissolve it in 3 ml of DMSO. Weigh 1.7mg EDC and dissolve it in 2ml DMSO, add the EDC solution to mPEG-COOH, and stir for 3 hours. Weigh the fifth generation PAMAM dendrimers (G5.NH 2 ) 13mg was dissolved in 4mL DMSO to prepare a solution with a concentration of 10.0mg / mL. According to mPEG-COOH / G5.NH 2 The molar ratio is 10 / 1, add the activated mPEG-COOH solution to G5.NH 2 solution, stirred magnetically at room temperature, and reacted for 3 days. Get sample G5.NH 2 -mPEG2k 10 solution. Add 171.49 μL HAuCl dropwise to the above solution 4 Aqueous solution (30mg / mL), mix and stir for 30min, then quickly add 23.6μL of 10mg / mL NaBH 4 Solution, reduction reaction 3h. After the reaction, the reaction product was transferred to a dialysis bag with a molecular weight cut-off of 14,000, and dialyzed in distilled water for 3 days (3 times a day). Freeze-d...
Embodiment 2
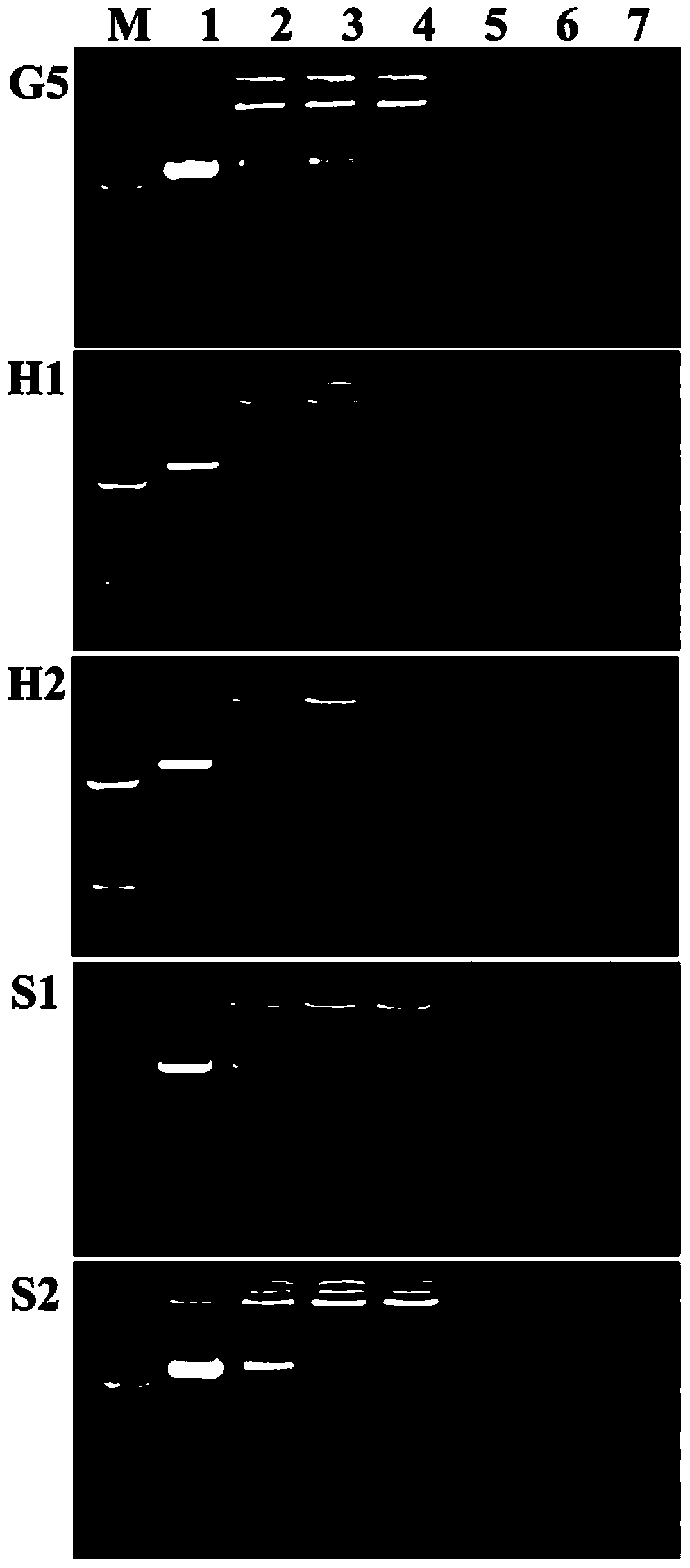
[0066] Gel electrophoresis experiments were used to test the ability of the vector to complex pDNA or siRNA and to determine the N / P ratio at which the material was able to fully compress pDNA or siRNA. Firstly, the carrier material and 1 μg pDNA or siRNA complexes were configured to form complexes at different N / P (0.125, 0.25, 0.5, 1, 2, 5) ratios, and reacted at room temperature for 20-30 minutes. Prepare ethidium bromide (0.1 μg / mL) agarose gel (1.0% w / v), and place at room temperature until the agarose gel solidifies. Using naked pDNA or siRNA as a control, add the corresponding vector / pDNA or vector / siRNA complexes to the wells of the agarose gel respectively, with a voltage of 80V and a time of 30min. Use a gel imager to analyze the migration of pDNA or siRNA in the gel. The results are shown in Figures 3(a) and 3(b). It shows that all four vectors can completely compress pDNA and siRNA when N / P>0.5, and this result also provides a reference for the selection of N / P i...
Embodiment 3
[0068] 5μg pDNA or siRNA (N / P=1, 2.5, 5, 10) were mixed with four vectors and G5.NH 2 After complex formation, the volume was made up to 1 mL with deionized water. The particle size and surface potential of the composite were measured using a Malvern laser particle size analyzer (Malvern, UK, 633nm laser), and the results are shown in Figure 4(a) and 4(b). It shows that the surface potentials of the complexes are all positive, indicating that the complexes can be easily combined with cells through electrostatic. The particle size of the complexes is about 200nm, which is conducive to the phagocytosis of cells.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com