A kind of gene carrier system and its preparation method
A gene carrier and system technology, applied in the field of biological carriers, can solve the problems of low transfection efficiency, affecting the binding efficiency of the carrier system and cells, etc., and achieve the effects of reducing cytotoxicity, improving binding efficiency, and improving turnover ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
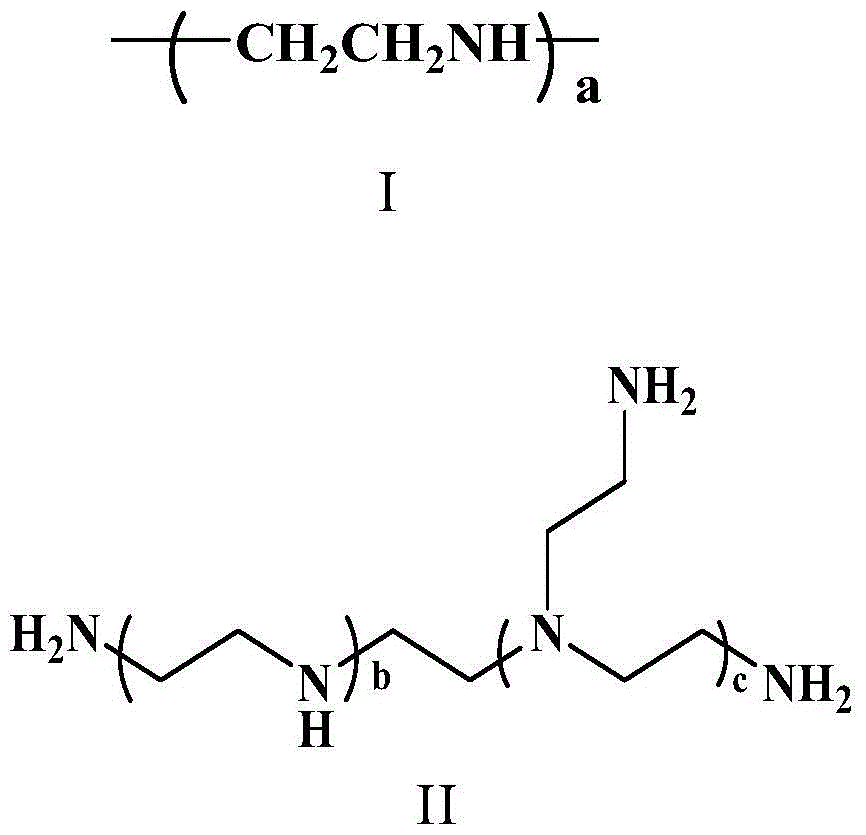
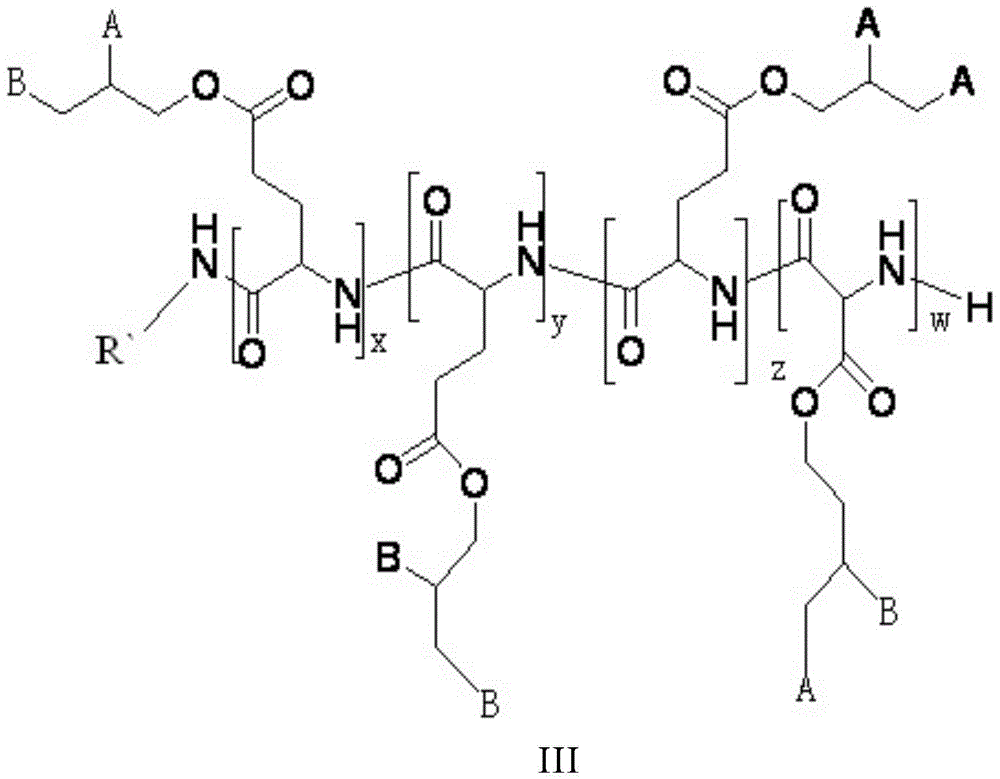
[0051] ③ The preparation method of the polymer α is: dissolve PEI-PPLG-g-(MEA-BOC / MAA), add acid for deprotection, then settle and drain, and obtain the polymer α through dialysis purification. The reaction has no requirement on the dissolved solvent, preferably chloroform or DMF, more preferably DMF; the reaction has no requirement on the type of acid added, preferably trifluoroacetic acid and hydrochloric acid; the reaction has no requirement on temperature, preferably 20-40°C . The molecular cutoff during dialysis is preferably 1,000 to 5,000.
[0052] In the amino acid polymer of the present invention, when the amino acid polymer is the small molecule alkylamine, the ring-opening polymerization of γ-propynyl-L-glutamic acid-N-internal carboxylic acid anhydride is initiated to obtain polyglutamate and 2- When tert-butoxycarbonyl aminoethanethiol and mercaptoacetic acid react and take off the polymer obtained by tert-butoxycarbonyl protection, i.e. polymer β, it is preferab...
Embodiment 1-8
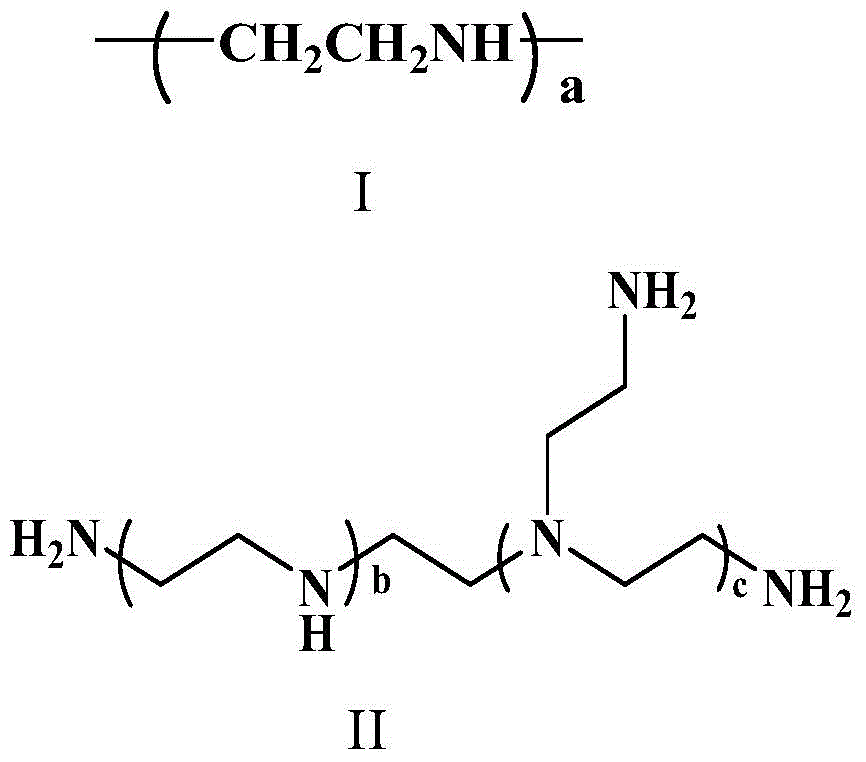
[0081] The ring-opening polymerization of γ-propynyl-L-glutamic acid-N-internal carboxylic acid anhydride was initiated by polyethyleneimine to obtain polyglutamate, and then reacted with 2-tert-butoxycarbonylaminoethanethiol and thioglycolic acid to remove The polymer obtained by tert-butoxycarbonyl protection, i.e. polymer α, its preparation method is as follows:
[0082] ①Preparation of PLG: Disperse 20g of L-glutamic acid in 30mL of propynyl alcohol, and add 8mL of concentrated sulfuric acid drop by drop under stirring in an ice bath. After half an hour of reaction, the reaction was carried out at room temperature of 28°C for 12h. The product was neutralized with 28 g of sodium bicarbonate in ice water and stirred until a solid precipitated. Filter and dry. The optimized experiment requires recrystallization and purification: heating and dissolving in water once, then cooling to precipitate, suction filtration, and drying. Gamma-propynyl-L-glutamate (PLG) was obtained. ...
Embodiment 9-16
[0097] The ring-opening polymerization of γ-propynyl-L-glutamic acid-N-internal carboxylic acid anhydride is initiated by a small molecule alkylamine to obtain polyglutamate, which is then reacted with 2-tert-butoxycarbonylaminoethanethiol and thioglycolic acid to remove The polymer obtained by tert-butoxycarbonyl protection, i.e. polymer β, its preparation method is as follows:
[0098] ①Preparation of PLG: Disperse 20g of L-glutamic acid in 30mL of propynyl alcohol, and add 8mL of concentrated sulfuric acid drop by drop under stirring in an ice bath. After half an hour of reaction, the reaction was carried out at room temperature of 28°C for 12h. The product was neutralized with 28 g of sodium bicarbonate in ice water and stirred until a solid precipitated. Filter and dry. The optimized experiment requires recrystallization and purification: heating and dissolving in water once, then cooling to precipitate, suction filtration, and drying. Gamma-propynyl-L-glutamate (PLG) ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com