Chemically modified graphene
A graphene and graphite technology, applied in the field of graphene derivatives, can solve the problems of biomolecular denaturation and separation process, complication, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
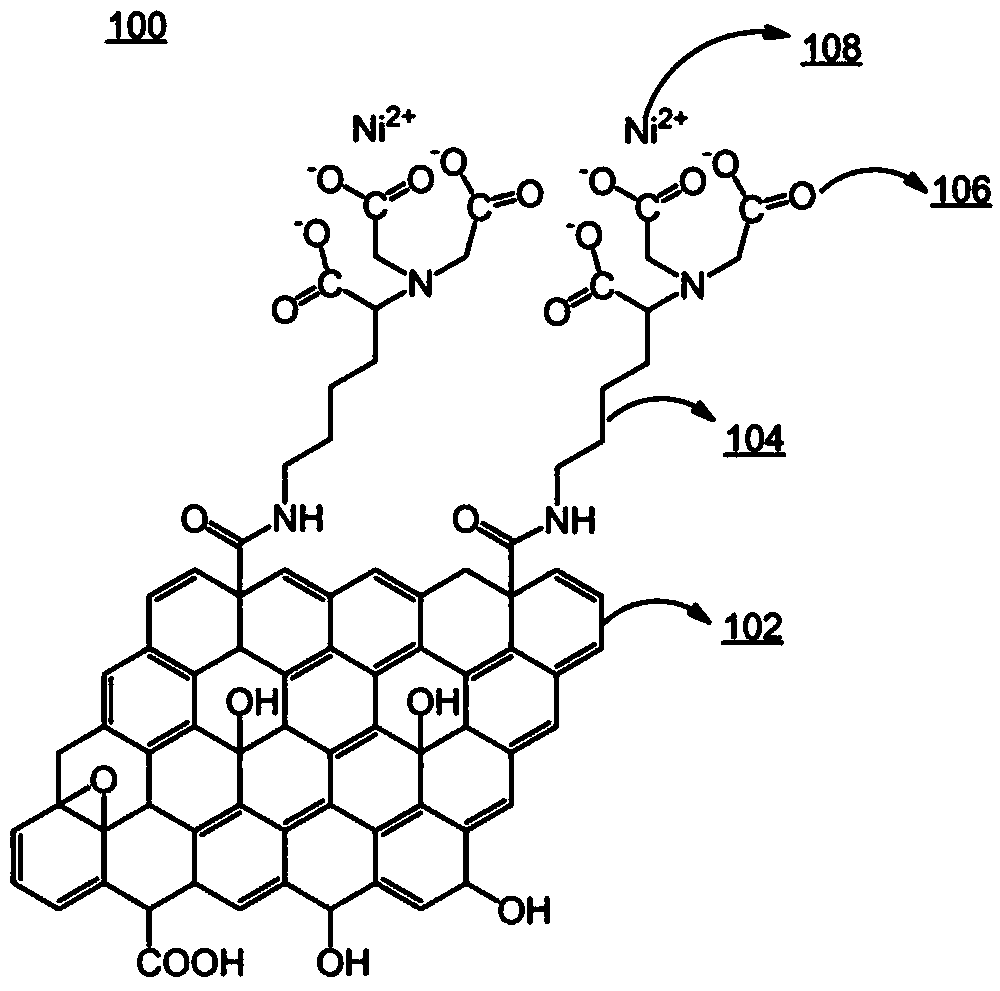
[0091] Embodiment 1: the preparation of the graphene derivative that contains nickel ion
[0092]
[0093] Graphene derivatives containing nickel ions were prepared as follows: the starting material is a suspension containing graphene oxide (GO) with an oxygen content of 8 mol% to 35 mol%, courtesy of Rutgers University Provided by the Nanomaterials and Devices Research Group. All preparations were performed at room temperature. Specifically, a mixture of 10 mM N-hydroxysuccinimide (NHS) and 10 mM N-ethyl-N'-(3-dimethylaminopropyl)-carbodiimide (both relative to those on GO 10 mL solution containing 15-20 vol% GO was treated for 1 hour. The suspension was then centrifuged at 21,000×g for 5 minutes, and the precipitate was washed with ultrapure water to obtain a graphene derivative containing an activated carboxyl group. Then with an excess (10 mL) of 150 mM N a ,N a - Treat the washed precipitate with a solution of bis(carboxymethyl)-L-lysine hydrate (pH=9.8) for 1 h...
Embodiment 2
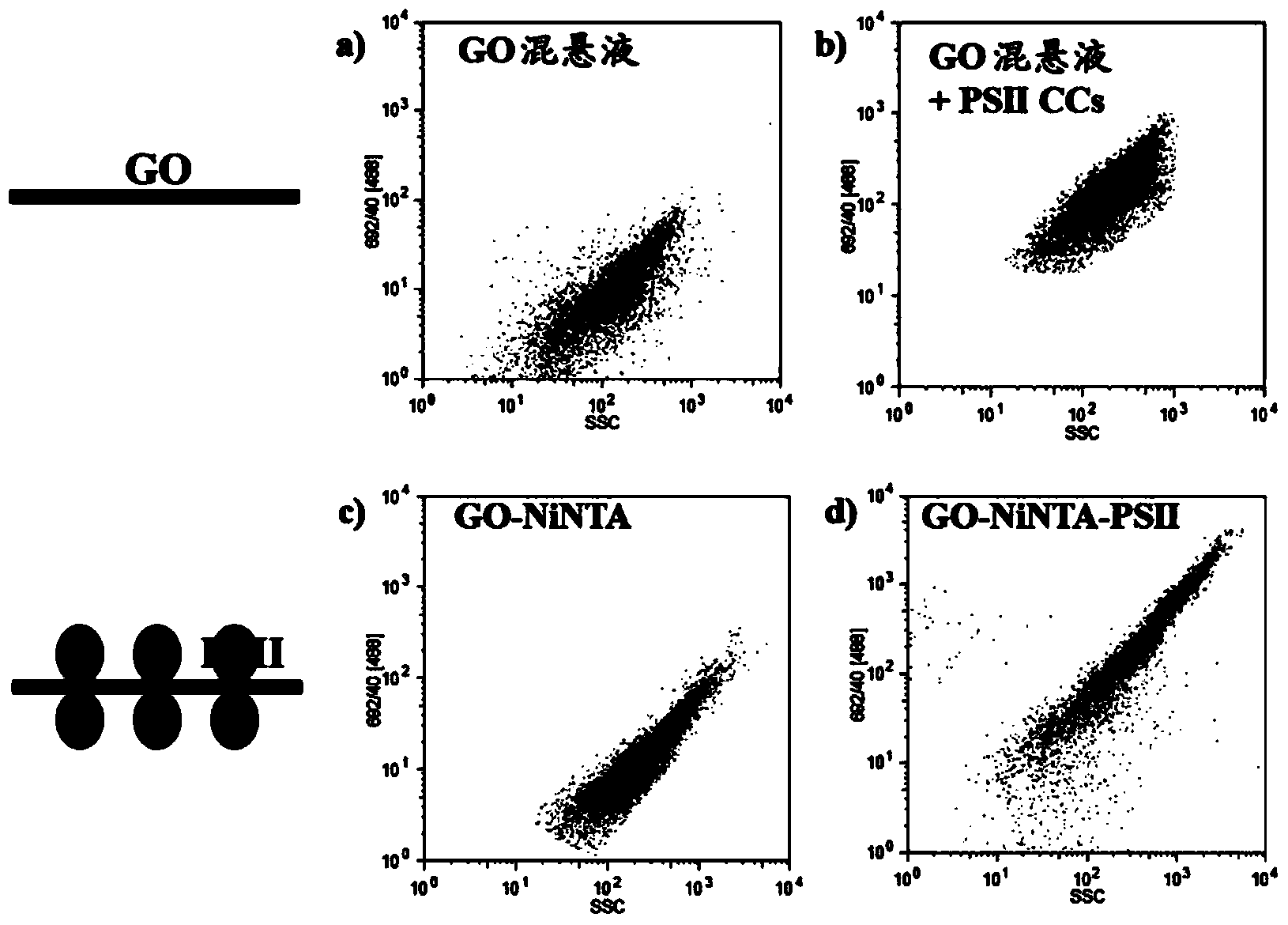
[0095] Example 2: In suspension, the PSII core complex is immobilized on GO-Ni-NTA IMAC resin superior
[0096] A His-tagged photosystem II core complex (PSII CC) protein on the protein domain CP47 was isolated from the thermophilic cyanobacterium Thermosynechococcus elongatus by standard protocols. The PSII CC protein is a dimeric protein with a physical size of 20.5nm(L)×11.0nm(W)×10.5nm(D) and a molecular weight of 680kDa. The His-tagged PSII CC protein was incubated in suspension containing GO-Ni-NTA resin (prepared in Example 1) for 20 to 60 minutes.
[0097] Coordination of PSII CC proteins to the resin was verified using flow cytometry and the protein loading capacity of the resin was measured. Using an interrogating laser at 488nm, the flow cytometer allows real-time and simultaneous measurement of fluorescence (both edge scatter (SSC) and forward scatter (FSC)) at 692nm. In the presence of a sheath fluid, individual particles undergo hydrodynamic aggregation. S...
Embodiment 3
[0102] Example 3: PSI core complex protein immobilized on GO-Ni-NTA film
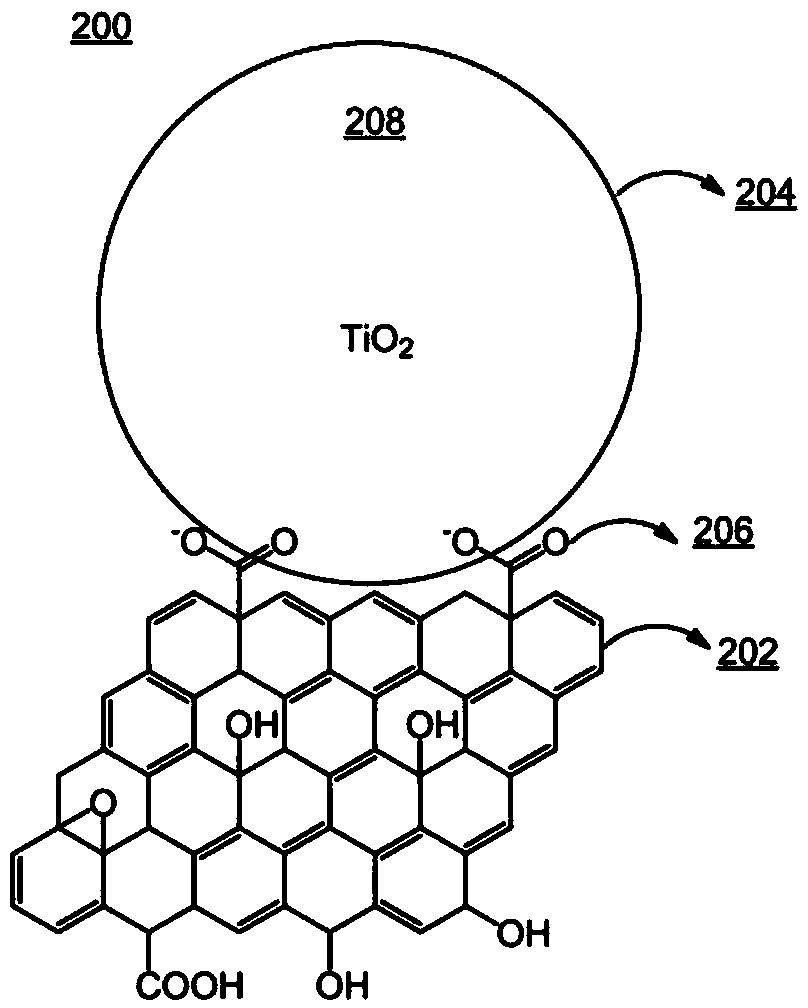
[0103] Functionalization using Ni-NTA groups directly at 1 cm 2 The gold-plated silicon support, which was further coated with GO with an oxygen content of 8-35 wt%, was performed on top of the Si-Au-GO support. The surface treatment process was carried out at room temperature. Specifically, the described Si-Au-GO support for 1 h to activate the carboxyl groups on the GO. After washing the activated Si-Au-GO support three times with ultrapure water, an excess (3 mL) of 150 mM N a ,N a - Bis(carboxymethyl)-L-lysine hydrate (pH=9.8) solution to treat it for 1 hour. The Si-Au-GO support thus obtained was washed three times with ultrapure water to obtain a support containing GO with nitrilotriacetic acid moiety. Then with an excess (3 mL) of 100 mM NiSO 4 Solution processing the washed support results in a support containing GO with nickel ions. In various steps, functionalization was confirmed by...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com